Ibuprofen-Induced Hepatotoxicity in Patients With Chronic Hepatitis C
ABSTRACT
Hepatitis C is a common chronic infection. Nonsteroidal anti-inflammatory drugs are commonly ingested both over-the-counter and by prescription. This case report describes three cases where ibuprofen use leads to a marked rise in hepatic transaminases with one case repeating on rechallenge. These cases support the recommendation of acetaminophen over nonsteroidal anti-inflammatory drug use in patients with chronic hepatitis C. (Am J Gastroenterol 1998; 93:1563-1565)
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most frequently prescribed medicines in the world, in addition to widespread over-the-counter use (1). NSAIDs are a well known cause of liver dysfunction. Aspirin is intrinsically toxic to the liver, whereas most other NSAIDs induce injury to the liver by an idiosyncratic reaction (2). Many reports confirm that NSAIDs cause elevation of aminotransaminases with convincing episodes after rechallenge (1-8). A rise in transaminases above 1000 U/L (U/L) occurs rarely, in less than 10% of cases (2).
Hepatitis C virus (HCV) infection is a major health problem around the world with an estimated 4 million cases in the United States alone. At least 50% of those infected with HCV have clinically apparent disease, with elevated serum aminotransaminases and histological activity of chronic hepatitis (9-12).
Hepatitis C-infected individuals are not immune to the usual musculoskeletal and headache complaints that are prevalent in our society. In fact patients, infected with hepatitis C frequently complain of arthralgias and have an increased incidence of autoimmune diseases including cryoglobulinemia that predispose to arthralgias (13). For these reasons, those infected with HCV may use either prescribed NSAIDs or over-the-counter NSAIDs.
The incidence of injury from NSAID use in chronic liver conditions such as hepatitis C is unknown. Large cohort studies defining the incidence in the population have excluded those with chronic liver diseases (4). We report three cases of NSAID-induced flares of hepatitis in patients with chronic HCV infection. No previous reports have described an association between a rise in liver transaminases in those with HCV and ingestion of NSAIDs.
CASE REPORTS
The first patient was a 33-yr-old Caucasian man who was discovered to have abnormal liver transaminases during an infertility evaluation. Studies revealed a serum level HCV-RNA of 2,400,000 copies/ml. The risk factor for acquisition of HCV was receiving a tattoo. Percutaneous liver biopsy demonstrated chronic active hepatitis with a Knodell score of six of 22. Baseline for the liver transaminases included an aspartate aminotransferase (AST) of 63 U/L and an alanine aminotransferase (ALT) of 212 U/L. A sudden rise of the AST/ALT to 459/1209 U/L was noted after ingesting 800 mg of ibuprofen, obtained by prescription, b.i.d. for 3 days for neck pain. Evaluation included negative hepatitis B surface antigen and hepatitis A serology. Three months after ibuprofen ingestion, the AST/ALT (123/293 U/L) returned to baseline. Six months later, a second rise in the transaminases was noted to AST/ALT of 524/1285 U/L. Ibuprofen 800 mg. b.i.d. had been ingested for 2 days during the week before these elevations for shoulder and neck pain. Three months after discontinuation of ibuprofen, the transaminases again returned to baseline (AST/ALT 80/130 U/L) (Fig. 1).
The second patient was a 44-yr-old Caucasian man with a history of intravenous drug use at age 19. Abnormal liver enzymes (AST/ALT 180/287 U/L) on routine screening prompted an evaluation. Presence of HCV infection was documented by HCV antibody with the second generation ELISA assay, and confirmed by RT-PCR (HCV-RNA 8,400,000 copies/ml). Percutaneous liver biopsy showed chronic active hepatitis and cirrhosis consistent with hepatitis C. After ingestion of ibuprofen obtained over-the-counter 600 mg b.i.d. four times a week for neck pain, a sharp increase in the AST/ALT (523/1238 U/L) occurred. After discontinuation of the ibuprofen transaminases returned to baseline within 2 months (AST/ALT 105/166 U/L)(Fig. 1). Evaluation of other etiologies including hepatitis A and B was unrevealing.
The third patient was a 34-yr-old Caucasian man diagnosed with HCV infection after elevated liver tests (AST/ALT 63/118) were found during a routine insurance physical. The risk factor was a blood transfusion 20 yr before. The HCV antibody was positive and confirmed with HCV-PCR (20,000,000 copies/ml). Percutaneous liver biopsy showed chronic hepatitis with fibrosis. After ingestion of long acting ibuprofen 800 mg every 12 h for wrist pain, obtained by prescription, the transaminases suddenly rose to AST/ALT 597/1577. Upon discontinuation of the ibuprofen, the liver tests returned to baseline within 2 months (AST/ALT 58/108 U/L). Hepatitis A and B were excluded and no other etiology could be found.
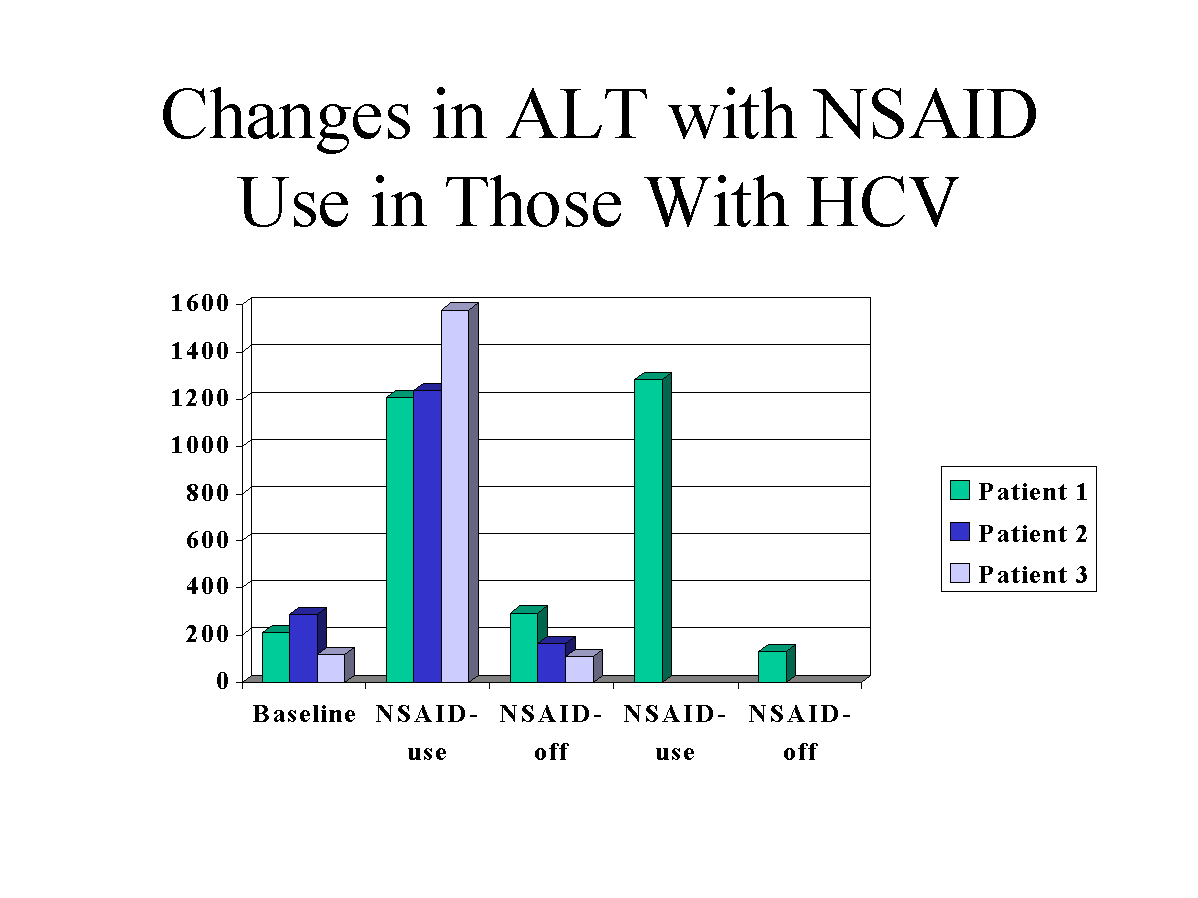
FIG.1. Changes in ALT with NSAID use in those with HCV.
DISCUSSION
We describe three patients with chronic hepatitis C infection who developed a greater than 5-fold elevation of the liver transaminases after ingestion of ibuprofen. All patient’ transaminases returned to baseline within 3 months of discontinuation of the ibuprofen. One patient exhibited a reproducible rise in the transaminases with rechallenge (Fig. 1). Other potential causative factors were excluded, including coinfection with hepatitis A and B and alcohol use.
Liver injury from ibuprofen use has rarely been reported (14-16). Rheumatoid arthritis and systemic lupus erythematosus are thought to increase the risk of liver injury with ibuprofen (4,14,16). No previous reports are found that describe ibuprofen toxicity in chronic liver disease. The mechanism by which ibuprofen induces liver injury is thought to be immunogenic or metabolic idiosyncrasy, but cases of overdoses with resultant liver injury suggests an intrinsic mechanism (17,18). The therapeutic doses that were taken in these cases suggests idiosyncrasy. The degree of abnormality in liver transaminases observed in these cases favors predilection and sensitivity to ibuprofen in those infected with HCV.
The transaminase pattern of elevation in chronic hepatitis C infection may vary but most commonly ranges from 1.5- to 3-fold over normal. An individual’s pattern of elevation can also vary through time and when monitored and plotted often appears “saw-toothed”. Variation usually is not more than double the baseline value, and tends to go up and down around some mean value. Changes from the mean point of greater than 5-fold are distinctly unusual and should prompt a search for other possible causes. Possible etiological factors to consider with such a rise includes a secondary infection with hepatitis A and B and alcohol use. Those infected with hepatitis C do not appear to be immune from reinfection from hepatitis C even of the same genotype. A careful review of the medications is often revealing for potential causes.
An under-appreciated cause of unexpected, sudden, steep rise in the liver transaminases is NSAIDs, as evidenced by these cases. In the three cases described, each patient reported the use of ibuprofen within 1 wk before a greater than 5-fold increase occurred in the liver transaminases. The fact that these three cases occurred with ibuprofen is alarming, because this drug is thought to be the safest NSAID in terms of liver toxicity (2,5). These three cases involved patients with a spectrum of HCV disease from relatively mild hepatitis to cirrhosis, and suggest that toxicity from ibuprofen in patients with HCV is not dependent on severity of disease.
Because of the high renin state that exists in cirrhosis, NSAIDs are known to reduce creatinine clearance secondary to marked reduction in prostaglandin E2 levels (2). Given this renal sensitivity and the possible gastrointestinal bleeding risk, NSAIDs are not usually recommended in cirrhotic patients. Frequently, acetaminophen is avoided in those with chronic liver diseases because of concern of intrinsic liver toxicity. NSAIDs are often prescribed instead for a variety of headaches or musculoskeletal complaints. No cases have been reported of hepatic injury occurring in patients with HCV, either with or without cirrhosis, and no active alcohol use when taking less than or equal to 2 g/24 h of acetaminophen (19). Based on this report, the recommendation on the use of analgesics to patients infected with HCV should be to avoid NSAIDs when possible and, when analgesics are needed, to take acetaminophen at a dose of less than or equal 2 g/24 h. If NSAIDs are deemed necessary, careful monitoring of the liver function tests should take place with monthly testing (at least) for the first 3 months of use and then every 3 months thereafter. Further study is needed of a large group of patients with hepatitis C to determine incidence of NSAID toxicity in this patient population and to develop a cost-effective approach to NSAID use and follow-up in this population.
REFERENCES
1. Carson JL, Willet LR. Toxicity of nonsteroidal anti-inflammatory drugs: An overview of the epidemiological evidence. Drugs 1993;46S:243-8.
2. Rabinovitz M, Van Thiel DH. Hepatotoxicity of nonsteroidal anti-inflammatory drugs. Am J Gastroenterol 1992;l87:1696-1704.
3. Carson JL, Strom BL, Duff A, et al. Safety of nonsteroidal anti-inflammatory drugs with respect to acute liver disease. Arch Intern Med 1993;153:1331-6.
4. Rodriguez LG, Williams R, Derby L, et al. Acute liver injury associated with nonsteroidal anti-inflammatory drugs and the role of risk factors. Arch Intern Med 1994;154:311-6.
5. Freeland GR, Northington RS, Hedrich DA, et al. Hepatic safety of two analgesics used over the counter: Ibuprofen and aspirin. Clin Pharmacol Ther 1988;43:473-9.
6. Banks AT, Zimmerman HJ, Ishak KG, et al. Diclofenac-associated hepatotoxicity: Analysis of 180 cases reported to the food and drug administration as adverse reactions. Hepatology 1995;22:820-7.
7. Manoukian AV, Carson JL. Nonsteroidal anti-inflammatory drug-induced hepatic disorders. Drug Safety 1996;15:64-71.
8. Brass EP. Hepatic toxicity of antirheumatic drugs. Cleve Clin J Med 1993;60:466-72.
9. Alter HJ. To C or not to C: These are the questions. Blood 1995;85:1681-95.
10. Tang E. Hepatitis C virus: a review. West J Med 1991;155:164-8.
11. Rubin RA, Falestiny M, Malet PF. Chronic hepatitis C: Advances in diagnostic testing and therapy. Arch Intern Med 1994;154:387-92.
12. Corny-Cantilena C, VanRaden M, Gibble J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. N Engl J Med 1996; 334:1691-6.
13. Agnello V. Mixed cryoglobulinemia and hepatitis C virus. Hospital Practice 1995;3:35-42.
14. Sonnenblack M, Abraham AS. Ibuprofen hypersensitivity in systemic lupus erythematosus. Br Med J 1978;1:619-20.
15. Bravo JF, Jacobsen MP, Mertens BF. Fatty liver and pleural effusion with ibuprofen therapy. Ann Intern Med 1977;87:200-1.
16. Stempel DA, Miller JJ. Lymphopenia and hepatic toxicity with ibuprofen. J Pediatr 1977;90:657-8.
17. Fry WF, Seeff LB. Hepatotoxicity of analgesics and anti-inflammatory agents. Gastroenterol Clin North Am 1995;24:875-905.
18. Lee CY, Finkler A. Acute intoxication due to ibuprofen overdose. Arch Pathol Lab Med 1986;110:747.
19. Zimmerman HJ, Maddrey WC. Acetaminophen hepatotoxicity with regular intake of alcohol: Analysis of instances of therapeutic misadventure. Hepatology 1995;22:767-73.




