HEPATOLOGY, March 1998, p. 868-872, Vol. 27, No. 3
Original Articles
Slow Progression Rate of Fibrosis in Hepatitis C Virus Patients With Persistently Normal Alanine Transaminase Activity
Philippe Mathurin1,3, Joseph Moussalli1, Jean-François Cadranel2, Vincent Thibault1, Frédéric Charlotte1, Patrice Dumouchel2, Alain Cazier2, Jean-Marie Huraux2, Bruno Devergie2, Michel Vidaud3, Pierre Opolon1, and Thierry Poynard1,3
From 1 Services d’HépatoGastroentérologie, d’Anatomie-Pathologique et de Virologie, Groupe Hospitalier Pitié-Salpêtrière; 2 Services d’HépatoGastroentérologie, d’Anatomie-Pathologique et de Virologie Hôpital Laennec Creil; and 3 CNRS URA 1484 Paris, France
ABSTRACT
In hepatitis C virus (HCV) patients with persistently normal alanine transaminase (ALT), the progression rate of fibrosis is unknown. The aims of this study were: 1) to compare HCV patients with normal ALT (group I) with HCV patients with elevated ALT (group II) matched on independent factors associated with fibrosis; and 2) to assess the progression rate of fibrosis. One hundred two HCV patients were included in each group. Histological lesions were staged using the METAVIR score. We defined fibrosis progression per year as the ratio of the fibrosis stage in METAVIR units to the duration of infection. In group I, ALT values were normal, and lower than in group II (25 vs. 127 IU/L; P < .0001). HCV RNA was present less frequently in group I (66% vs. 97%; P < .0001). There were no significant differences for viremia and genotypes. Histological activities were lower in group I (0.6 vs. 1.38; P < .0001). The stage of fibrosis was lower in group I (0.95 vs. 1.8; P < .001). The median progression rate of fibrosis was lower in group I (0.05 vs. 0.13; P < .001). In group I, after exclusion of negative HCV-RNA patients, the median progression rate of positives remained lower (0.05 vs. 0.13; P < .001). In group I, all cirrhotic patients (n = 3) were heavy drinkers. HCV patients with normal ALT showed weaker histological activity and lower fibrosis scores, and the progression rate of fibrosis was twice as slow as in HCV patients with elevated ALT. In these patients, severe fibrosis was associated with high alcohol consumption. (HEPATOLOGY 1998;27:868-872.)
INTRODUCTION
Hepatitis C virus (HCV) is the main cause of chronic viral liver disease in western countries. In approximately 70% of patients, HCV infection runs a chronic course, with histological lesions that can worsen and lead to cirrhosis in 20% of cases. Alanine transaminase (ALT) is commonly used as a marker of hepatic inflammation and damage in HCV infection. However, it has been established that ALT values are poorly correlated with histological outcome.1-3
Fluctuating ALT levels characterize chronic hepatitis C, but, in a few cases of infected HCV patients, ALT values are persistently normal. Controversies persist concerning virological, epidemiological, and histological characteristics of HCV patients with persistently normal ALT levels. The discrepancies between previous studies may be caused by several factors involving: 1) inclusion of patients with near-normal ALT values in addition to patients with strictly normal ALT values4; 2) ranges in the number of ALT values obtained5-10; 3) the absence of data concerning alcohol consumption5-14; 4) a limited number of patients; and 5) the absence of comparison with control patients matched on independent factors associated with liver fibrosis, such as age, sex, alcohol consumption, and duration of infection.1 These reasons probably contributed to the absence of definite conclusions concerning HCV patients with normal ALT.
Controversies exist concerning histological findings in patients with normal ALT levels. Some authors have shown that, in this group of patients, liver histology is virtually normal.8 In contrast, other authors have shown that HCV viremia in persons with normal ALT is consistently associated with liver damage. 5,6 These studies do not focus on fibrosis, which is clinically more important than the activity grade.1
One clinical problem in patients with HCV infection is to determine the progression rate of fibrosis.1 It is not known whether this progression is different in patients with normal ALT activity in comparison with patients with elevated ALT. It is also not known whether these patients with persistently normal ALT serum activities truly differ from a population of patients with abnormal ALT matched for the main characteristics potentially associated with fibrosis, such as age, sex, ethnic origin, alcohol consumption, and duration of infection. In the present study, the standardized estimate of fibrosis was based on the classification of fibrosis into four stages (F0 to F4) that we had previously validated.15
Thus, the aims of the present study were: 1) to evaluate demographic, histological, and virological features of patients with normal ALT activity; 2) to assess the fibrosis progression rate in such patients; and 3) to compare these parameters to matched control patients with elevated ALT activity.
PATIENTS AND METHODS
Patients. Between January 1992 and December 1996, among 1,353 patients with anti-HCV-positive antibodies referred to our liver units, 102 untreated patients with repeatedly normal ALT activity (7.5% of the total cohort) were prospectively included in this study (group I). ALT measurements were performed on a Hitachi 911 automat using Boehringer Mannheim reagents. The common threshold of 45 IU/L was used as the upper normal range. This threshold corresponded to the mean + 2 SD of a control population given by the manufacturer and after exclusion of the 5% extreme values. In all patients, ALT was measured at least three times during the 6 previous months. All subjects underwent physical examination, liver ultrasonography, and routine liver function tests (i.e., ALT, aspartate transaminase, ![]() -glutamyl transpeptidase [GGT], total serum bilirubin, and prothrombin time). Patients were not selected for specific risk factors. We systematically determined alcohol consumption after questioning the subjects on admitted daily alcohol intake during the last 5 years. Patients with other forms of liver disease, including hepatitis B virus, autoimmune hepatitis, and genetic liver disease, were excluded. Patients with anti-human immunodeficiency virus antibodies, immunosuppressive treatment, or who were under renal replacement therapy were also excluded. The patients with normal ALT values were compared with 102 patients (group II) with elevated ALT matched for age, sex, ethnic origin, alcohol consumption, and duration of infection. Demographic data of the two groups are shown in table 1.
-glutamyl transpeptidase [GGT], total serum bilirubin, and prothrombin time). Patients were not selected for specific risk factors. We systematically determined alcohol consumption after questioning the subjects on admitted daily alcohol intake during the last 5 years. Patients with other forms of liver disease, including hepatitis B virus, autoimmune hepatitis, and genetic liver disease, were excluded. Patients with anti-human immunodeficiency virus antibodies, immunosuppressive treatment, or who were under renal replacement therapy were also excluded. The patients with normal ALT values were compared with 102 patients (group II) with elevated ALT matched for age, sex, ethnic origin, alcohol consumption, and duration of infection. Demographic data of the two groups are shown in table 1.
| table 1. Clinical and Biochemical Features |
Virological Studies. Anti-HCV was determined using a commercial ELISA 3 (Ortho Diagnostic System, Raritan, NJ) and was confirmed by immunoblot using the RIBA 3 assay (Chiron Corporation, Emeryville, CA) in 172 cases, or using Deciscan (Institut Pasteur Paris, France) in 30 cases. Serum samples were collected for detection, quantitation, and typing of HCV RNA. HCV RNA was detected by polymerase chain reaction with the Amplicor Roche Diagnostic System (Hoffmann-LaRoche, Basel, Switzerland). Quantification was performed by branched DNA (bDNA) with the quantiplex HCV-RNA 2.0 Chiron assay (bDNA 2.0, Chiron). The detection threshold of bDNA was 2 × 105 Eq gen/mL. HCV genotyping was performed either with Innogenetics Inno-Lipa or with fluorescent competitive oligonucleotide primers.16 HCV quantification was performed in 62 unselected patients from group I and 60 unselected patients from group II. HCV genotyping was assessed in 63 patients from group I and 74 patients from group II.
Liver Biopsy Studies. Patients underwent percutaneous liver biopsy with a Hepafix needle (Hepafix B, Braun Melsungen AG, Germany) or transvenous liver biopsy. A sample of each biopsy was used for histological examination by light microscopy. Liver biopsy sections were formalin-fixed, paraffin-embedded, and stained routinely with hematoxylin-eosin. They were evaluated by two pathologists who were unaware of the patients’ clinical and laboratory data. The specimens were evaluated according to the METAVIR score. 15,16 Activity was graded according to the intensity of necroinflammatory lesions: 0 = no activity, 1 = mild activity, 2 = moderate activity, and 3 = severe activity. The stage of fibrosis was assessed on a 5-point scale: 0 = no fibrosis, 1 = portal fibrosis without septa, 2 = portal fibrosis with few septa, 3 = portal fibrosis with many septa, 4 = cirrhosis. In a previous study, assessment of fibrosis by the METAVIR scoring system showed a substantial interobserver degree of concordance (among 10 observers) with a ![]() value of 0.78.15 Progression per year of liver fibrosis was assessed in patients in whom the duration of infection was known. We defined fibrosis progression per year (unit F-METAVIR per year) as the ratio between the fibrosis stage in METAVIR units and the duration of infection in years.1
value of 0.78.15 Progression per year of liver fibrosis was assessed in patients in whom the duration of infection was known. We defined fibrosis progression per year (unit F-METAVIR per year) as the ratio between the fibrosis stage in METAVIR units and the duration of infection in years.1
Statistical Analysis. Statistical methods included qualitative data ![]() 2 tests, Fisher’s Exact test, and, for quantitative data, the t test and nonparametric tests (Mann-Whitney). Quantitative variables were expressed as means ± SD and median with its 95% confidence interval. The median was used when the distribution was not normal. The Pearson linear correlation coefficient was assessed between age, alcohol consumption, bDNA level, duration of infection, and histological stages.
2 tests, Fisher’s Exact test, and, for quantitative data, the t test and nonparametric tests (Mann-Whitney). Quantitative variables were expressed as means ± SD and median with its 95% confidence interval. The median was used when the distribution was not normal. The Pearson linear correlation coefficient was assessed between age, alcohol consumption, bDNA level, duration of infection, and histological stages.
RESULTS
Clinical and Biological Data. The main clinical and biological features of the two groups are given in table 1. There were no significant differences between the two groups for age, sex ratio, alcohol consumption, or duration of contamination. ALT and GGT activities were significantly lower in group I than in group II. There were no significant differences between the two groups for other biochemical variables. Eleven patients in groups I and II had alcohol consumption above 50 g/d: 130 ± 62 g/d versus 100 ± 50 g/d, respectively (not significant).
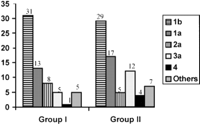
Virological Data. HCV RNA detected by polymerase chain reaction was found more frequently in group II than in group I (table 2). Among HCV-RNA-positive patients, regardless of their group, there were no significant differences in the level of viremia (table 2) or in genotypes (Fig. 1). Genotype 1b was most frequent in both groups (45% of cases). In group I, there were no significant differences between HCV-RNA-positive patients and HCV-RNA-negative patients for the Riba pattern according to reactivity against C100-p (79% vs. 79%), C33-c (98% vs. 96%), C22-p (98% vs. 93%), or NS-5 (67% vs. 57%). In group I, anti-HCV titers (determined by enzyme-linked immunosorbent assay) were significantly higher in HCV-RNA-positive patients than in HCV-RNA-negative patients: 5 ± 0.37 vs. 4.4 ± 1.3; P = .02. In group I, HCV-RNA-positive patients had higher ALT levels than HCV-RNA-negative patients: 27 ± 8 vs. 18 ± 8 U/L; P < .0001.
| table 2. Histological and Virological Features |
|
|
Fig. 1. Distribution of genotypes in group I and group II. HCV genotyping was assessed in 63 unselected patients from group I and 74 unselected patients from group II. |
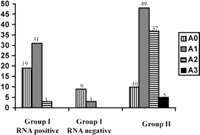
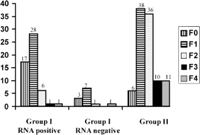
Histological Data. A total of 67 and 101 patients in groups I and II, respectively, underwent liver biopsies. In group I, there were no differences between patients who did not undergo liver biopsy and patients who underwent liver biopsy for the number of ALT measurements, GGT activity, bDNA level, age, alcohol consumption, and duration of infection. The only differences were that patients who did not undergo liver biopsy had lower ALT activity (21.6 ± 9.9 vs. 26 ± 7.9; P < .001) and were less frequently HCV-RNA-positive (20% vs. 80%; P < .0001). Histological activity and fibrosis stages were significantly lower in group I than in group II (table 2). Distribution of fibrosis and activity stages are given in Figs. 2 and 3. Fibrosis progression per year evaluated in 54 and 83 patients (in whom duration of infection was known) from groups I and II, respectively, was significantly lower in group I than in group II (table 2). After exclusion of HCV-RNA-negative patients from group I, histological activity and fibrosis stages and progression rate of liver fibrosis remained significantly lower in group I than in group II (table 2). After exclusion of patients with daily consumption above 50 g (11 in group I and 11 in group II), mean activity (0.6 ± 0.6 vs. 1.4 ± 0.7; P < .0001), mean fibrosis scores (0.8 ± 0.7 vs. 1.8 ± 1; P < .0001), and median fibrosis progression per year (0.06 [95% CI: 0.04-0.07] vs. 0.11 [95% CI: 0.09-0.16]; P < .0001) remained lower in group I than in group II.
|
|
Fig. 2. Distribution of activity in group I RNA-positive, group I RNA-negative, and group II. |
|
|
Fig. 3. Distribution of fibrosis in group I RNA-positive, group I RNA-negative, and group II. |
Relationship in Group I Between Histological Features and HCV RNA, bDNA Level, Genotypes, ALT Activity, and Alcohol Consumption. HCV-RNA-positive patients showed a higher activity stage (0.7 ± 0.6 vs. 0.25 ± 0.45; P = .01) and a similar fibrosis score (1.1 ± 1.1 vs. 0.9 ± 0.8) in comparison with HCV-RNA-negative patients (table 2). There was no significant correlation between the bDNA level and ALT (IU/L) (r = .26; not significant), fibrosis stage (r = .2; not significant) or activity stage (r = .19; not significant). There was a significant correlation between the ALT level and the activity stage (r = .3; P < .02), whereas no correlation was found between the ALT level and the fibrosis stage (r = .05; not significant). There was no correlation between genotypes and histological features (data not shown). In the subgroup of patients with severe fibrosis (fibrosis score above 3, n = 4), heavy drinkers were more frequently found (3 of 4) than in the subgroup of patients without severe fibrosis (6 of 61, P = .007). In group I, 3 patients had cirrhosis and all were heavy drinkers. Patients with alcohol consumption above 50 g/d had a higher fibrosis stage than the others (1.8 ± 1.7 vs. 0.9 ± 0.7; P = .007), whereas there was no difference for the activity stage (0.6 ± 0.6 vs. 0.7 ± 0.7).
Sensitivity Analysis. In hepatitis C, periods of normal ALT can be interrupted by periods of elevated ALT activity. Therefore, to assess whether more frequent measurements selected another type of population, we divided patients from group I into two subgroups: patients (n = 41) with less than six measurements (mean: 4; range: 3-5) and patients (n = 61) with more than six measurements (mean: 8, range: 6-15). According to ALT measurements, there were no differences for age (43 ± 14 vs. 43 ± 14 ), duration of infection (15 ± 6.8 vs. 14.8 ± 8.2), ALT activity (25 ± 9 vs. 24 ± 9), GGT activity (27.6 ± 18.6 vs. 25.6 ± 21.7), bDNA level (61 ± 179 vs. 51 ± 89), fibrosis stage (0.9 ± 0.9 vs. 1 ± 1), mean activity stage (0.5 ± 0.5 vs. 0.7 ± 0.6), or median of fibrosis progression per year (0.05 [95% CI: 0-0.08] vs. 0.058 [95% CI 0-0.08]).
Patients from group II had significantly higher GGT than those from group I. To determine whether selection of patients from group II with elevated GGT values may represent a bias, we compared histological features of HCV-RNA-positive patients from group I with normal GGT values with those of patients from group II with normal GGT values. Comparison between these two subgroups of patients with normal GGT values showed that mean activity stage, mean fibrosis stage, and median of fibrosis progression per year remained significantly lower in patients from group I than those from group II: 0.74 ± 0.54 versus 1.3 ± 0.7; P< .001; 0.9 ± 0.75 versus 1.7 ± 0.96; P < .001; 0.05 (95% CI: 0-0.07) versus 0.12 (95% CI: 0.08-0.17); P < .001.
Because fibrosis progression per year was assessed in 55 patients from group I and 83 patients from group II, we performed a sensivity analysis strictly limited to paired cases from group I (n = 54) and from group II (n = 54). Progression of liver fibrosis per year remained lower in patients from group I than those from group II: 0.05 (95% CI: 0.04-0.07) versus 0.12 (0.07-016); P < .0001.
DISCUSSION
In the present study, we analyzed a high number of HCV patients with persistently normal ALT levels. We defined patients with persistently normal ALT on the basis of at least three normal ALT values obtained during the 6 previous months. It can be considered that this number of ALT values obtained may be insufficient. However, in this study, the mean number of ALT values obtained was six (table 1), and with this definition, among 1,353 HCV patients, only 102 patients were defined as patients with normal ALT values. Moreover, sensitivity analysis according to the number of ALT measurements did not reveal any differences between patients with less than six measurements and those with more than six measurements. Finally, in the literature, there is no clear definition of the number of ALT values required to consider a patient as having persistently normal ALT.
These patients were compared with HCV patients with elevated ALT matched on demographic data. The main findings were: 1) among patients with normal ALT, only 70% had detectable viremia; 2) HCV-RNA-positive patients without any histological lesions (A0F0 in the METAVIR scoring system), the so-called “healthy carriers,” represented 20% of the group with normal ALT in comparison with 4% of matched patients; 3) activity and fibrosis scores in the group with normal ALT were significantly lower than those of patients with elevated ALT even after exclusion of HCV-RNA-negative patients or of heavy drinkers, whereas virological features were similar (i.e., viral load and genotypes); 4) the progression rate of liver fibrosis was twice as slow in patients with normal ALT as in patients with elevated ALT even after exclusion of HCV-RNA-negative patients; and 5) severe fibrosis was associated with alcohol consumption in patients with normal ALT.
In previous studies, the absence of HCV RNA in sera from patients with normal ALT ranged from 10% to 90%. 11-14,18 In the present study, we found that HCV RNA was absent in one third of patients with persistently normal ALT. As suggested by the study of Shindo et al., which failed to detect HCV RNA in the livers of patients with negative HCV viremia, we postulate that approximately 30% of patients with strictly and persistently normal ALT level had spontaneously eliminated HCV, at least in serum. In the absence of a specific search for HCV RNA in the liver or in another reservoir such as lymphocytes, we cannot conclude that the virus has been definitively eradicated.
Controversies exist concerning histological findings in patients with normal ALT levels. In the present study using METAVIR, a histological classification for chronic viral hepatitis, 15,17 we found that 20% of patients were classified as A0F0 (so-called healthy carriers). In contrast, only 6% of normal ALT patients, as compared with 20% of elevated ALT patients, showed a fibrosis score higher than 2. In previous studies, strong heterogeneity was found in the prevalence of normal livers. 5,11 A recent review found that 15% of previously published patients (59 of 388) had normal liver histology, with substantial discrepancies between studies.19 The main discrepancies in these results may be caused by several factors: inclusion of patients with near-normal ALT values,4 ranges of number of ALT values obtained,5-10 and the absence of data concerning alcohol consumption.5-13 For instance, a recent study found 20% of cirrhosis in patients with normal ALT values; however, the authors did not give information concerning alcohol consumption, number of ALT values obtained, or mean ALT values.5 In our study, we found that all patients with cirrhosis were heavy drinkers in the group of patients with normal ALT. Therefore, we suggest that some variables such as alcohol consumption may explain in part the discrepancies between the studies.
A major concern lies in assessing the natural history of HCV-RNA-positive patients with normal ALT levels in comparison with the history of patients with elevated ALT levels. To our knowledge, no previous studies compared patients matched for age, sex, duration of infection, age at infection, ethnic origin, mode of contamination, or alcohol consumption, which are well-established variables associated with histological damage. For instance, some studies did not find significant histological differences between patients with normal or elevated ALT, 5,7,9 whereas others found a lower histological activity index in patients with normal ALT levels. 4,12,20 The absence of a control group with matched patients probably led to inconsistent data.
We found no significant differences between patients with normal ALT and patients with elevated ALT in terms of genotype distribution and HCV-RNA titer. Concerning genotype distribution, our results are in agreement with others, 5,12 in contrast to Silini et al., who found that the prevalence of genotype III was significantly higher in patients with normal or near-normal ALT, whereas genotype II was more frequently found in patients with biochemical features of chronic liver disease.4 In Silini’s study, no information was given concerning the ALT level and the inclusion of patients with ALT above the upper limit of the normal range. Concerning the HCV-RNA level, we did not find any differences between patients with normal ALT and patients with elevated ALT levels. Data from some previous studies showed significantly lower HCV viremia in patients with normal ALT, 17,21,22 whereas other studies found similar levels of bDNA.13
In the present study, one of the main results was the parametric estimate of liver fibrosis progression in patients with normal ALT as compared with patients with elevated ALT. We recently demonstrated, in a nonselected large group of HCV patients without treatment,1 that the median rate of fibrosis progression per year was 0.133 fibrosis units (95% CI: 0.125-0.143), which was similar to the 0.130 (95% CI: 0.100-0.160) median rate of fibrosis progression assessed in the present control group of patients with elevated ALT. The median rate of fibrosis progression in patients with normal ALT levels was 0.05 (95% CI: 0.040-0.070), suggesting a very low progression rate of liver fibrosis. The corresponding expected median time to cirrhosis for this group of slow fibrosis is F4/0.05, i.e., 80 years. After exclusion of patients with elevated GGT values, the progression rate of liver fibrosis remained significantly lower in patients with normal ALT. Therefore, we suggest that the natural history of HCV infection in patients with persistently normal ALT is associated with a low risk of developing severe liver lesions, especially in patients without alcohol consumption. Therapeutic strategies should take these findings into account.
Footnotes
Abbreviations: HCV, hepatitis C virus; ALT, alanine transaminase; GGT, -glutamyl transpeptidase; bDNA, branched DNA.
Supported by a grant from the Association pour la Recherche contre le Cancer, France
Received August 20, 1997; accepted November 13, 1997.
Address reprint requests to: Dr. P. Mathurin, Service d’HépatoGastroentérologie, Groupe Hospitalier Pitié-Salpêtrière, 47-83 boulevard de l’Hôpital, 75651 Paris, France. Fax: 33-1-45-86-20-22.
Copyright © 1998 by the American Association for the Study of Liver Diseases.