HEPATOLOGY, May 1998, p. 1200-1206, Vol. 27, No. 5
Original Articles
Decreased Muscle Strength in Patients With Alcoholic Liver Cirrhosis in Relation to Nutritional Status, Alcohol Abstinence, Liver Function, and Neuropathy
Henning Andersen1, Mette Borre2, Johannes Jakobsen1, Per Heden Andersen2, and Hendrik Vilstrup2
From the Departments of 1 Neurology and 2 Medicine V (Hepatology and Gastroenterology), Aarhus University Hospital, Aarhus, Denmark
ABSTRACT
To study motor function quantitatively in alcoholic liver cirrhosis muscle strength, liver function, peripheral nerve function, and nutrition were assessed in 24 patients. Isokinetic strength of flexion and extension at elbow, wrist, hip, knee, and ankle and of shoulder abduction and adduction was evaluated and compared with findings in 24 matched healthy subjects. Degree of liver disease was assessed with the Child-Pugh score and the galactose elimination capacity (GEC). Nutritional status was evaluated with an estimation of lean body mass (LBM) from 24-hour urinary creatinine excretions. Peripheral nerve function was evaluated with neurological symptom and disability scores, nerve conduction studies, and quantitative sensory tests summed to obtain a neuropathy rank-sum score (NRSS) for each patient. Combined muscle strength at hip, knee, ankle, shoulder, elbow, and wrist were weakened with 34% (P < .005), 35% (P < .001), 35% (P < .01), 34% (P < .01), 29% (P < .01), and 29% (P < .02), respectively. The median Child-Pugh score was 7 (range, 5-12), and the median duration of alcohol abstinence was 90 days (range, 5-960 days). After multiple linear regression analysis including LBM, Child-Pugh score, GEC, duration of alcohol abstinence, and NRSS, only LBM was correlated to the strength at the knee (r = .79; P < .0001) and at the ankle (r = .63; P < .01). It is concluded that muscle strength is weakened substantially in alcoholic patients with liver cirrhosis and that weakness is related to the severity of malnutrition but not to the severity of liver disease, duration of alcohol abstinence, or neuropathy. (HEPATOLOGY 1998;27:1200-1206.)
INTRODUCTION
It is a classical clinical observation that patients with liver cirrhosis waste muscle mass. The wasting probably leads to motor dysfunction and disability, but the functional consequences are sparsely documented. It is likely that reduced protein intake and malnutrition contribute to the wasting, but loss of muscle mass also occurs in patients with normal dietary intake.1 Decreased muscle protein synthesis and increased myofibrillar degradation in cirrhotic patients could play a role as well. 2,3 Furthermore, the physical inactivity associated with severe liver disease probably contributes to the wasting. In addition to loss of muscle mass, motor dysfunction could result from biochemical and physiological abnormalities of the contractile properties and the characteristics of the sarcolemma. Thus, lowered concentrations of energy-rich phosphagens and magnesium have been reported in studies of biopsy tissues of cirrhotic patients. 4,5 At present, it is unknown whether these abnormalities lead to motor dysfunction.
In chronic alcoholics, some studies have reported a predominant type 2 fiber atrophy, indicating the existence of a chronic alcoholic myopathy. 6,7 In other studies, mitochondrial alterations were observed, whereas fiber-type proportions and dimensions remained normal.8 Considering the few and unspecific histological abnormalities, the existence of the entity alcoholic myopathy is not generally accepted. Furthermore, some investigators suggest that chronic impairment of muscles in alcoholic patients is caused exclusively by neurogenic atrophy.9 Alcoholic polyneuropathy is a predominant sensory disturbance during the initial stages. Later on, degeneration of motor nerves can result in denervation and, consequently, in wasting and weakness.10 In chronic alcoholics, a relation between motor neuropathy and muscle atrophy has not been established.
In patients with alcoholic liver cirrhosis, it is unknown whether muscle wasting and motor dysfunction are caused by metabolic, nutritional, or neuropathic abnormalities. Therefore, the aim of the present study was to evaluate the muscular performance in patients with alcohol-induced liver cirrhosis with standardized quantitative techniques in relation to nutritional status, liver function, duration of alcohol abstinence, and peripheral nerve function.
PATIENTS AND METHODS
Patients and Control Subjects
During a 14-month period, 24 patients with alcohol-induced liver cirrhosis who were referred to the Department of Hepatology and Gastroenterology were included in the study. Ten patients were studied shortly after the initial admission, and the other 14 patients were studied at subsequent hospitalizations. In 17 patients, the diagnosis of liver cirrhosis was based on biopsy findings. Liver biopsy was not possible in the remaining 7 patients, and consequently, diagnosis was made from clinical (ascites or hematemesis) and laboratory findings (hypoalbuminemia or hypoprothrombinemia). Patients were not included if they had encephalopathy at the time of study, severe cardiopulmonary disease, diabetes mellitus, other endocrine disorders, acute or chronic musculoskeletal disease, or any other neurological or psychiatric disturbances. For comparison, the motor performance of 24 healthy age-, sex-, height-, and weight-matched controls was recruited among hospital employees, blood donors, friends, and relatives. To calculate a predictive value of muscle strength at the ankle and knee, the performances of an additional 65 healthy subjects were included. All patients and control subjects gave informed consent to the study, which was approved by the local ethics committee.
Methods
Clinical Evaluation and Biochemical Measures. To exclude patients with encephalopathy and dementia, all patients were assessed with the Mini Mental State Examination.11 Patients were not included if the score was <27 (maximum score, 30). The clinical status of the patients was assessed according to the Child-Pugh classification (group A, B, and C), using measures of plasma bilirubin, plasma albumin, prothrombin time, and the presence or absence of ascites and hepatic encephalopathy.12 Plasma bilirubin values of <34 µmol/L, between 34 and 51 µmol/L, and greater than 51 µmol/L gave scores of 1, 2, and 3, respectively. Correspondingly, plasma albumin values of greater than 35 g/L, between 28 and 35 g/L, and <28 g/L resulted in scores of 1, 2, and 3. Prothrombin time of greater than 50%, between 30% and 50%, and <30% of normal scored 1, 2, and 3, respectively. No ascites resulted in a score of 1, and scores for mild and severe ascites were 2 and 3, respectively. Patients with encephalopathy were excluded from the study, and therefore, all patients scored 1 for encephalopathy. As described elsewhere, patients with a total score of 5 to 6 were categorized as group A, scores of 7 to 9 were group B, and scores of greater than 9 were group C.
The 24-hour urinary excretion of creatinine was determined from two collections. Serum levels of albumin, creatinine, magnesium, sodium, potassium, total calcium, iron, phosphate, bilirubin, methyl malonate, hemoglobin, leukocytes, thrombocytes, glucose, alanine aminotransferase, alkaline phosphatase, ![]() -glutamyl transferase, prothrombin, thyreotropin-stimulating hormone, erythrocyte folate, immunoglobulin G, carbamide, M-component, and amylase were measured with standard laboratory techniques. Galactose elimination capacity (GEC)13 was determined at the time of the study except for a few patients. Eventually, all patients were evaluated by a trained neurologist according to a neuropathy symptom score14 and a neurological disability score.15 The neuropathy symptom score includes symptoms of motor, sensory, and autonomic disturbances. Motor symptoms are complaints about weakness of proximal and distal muscle groups of upper and lower extremities. In addition, complaints of facial and eye muscle palsy and difficulties in chewing and swallowing are noted. Sensory symptoms include difficulties in identifying objects with hand and in the mouth, unsteadiness during walking, numbness or paresthesia, and pain of neuropathic origin. Autonomic symptoms are postural fainting, male impotence, loss of urinary control, and nightly diarrhea. The neurological disability score is obtained from the neurological examination and includes scores of muscle strength, tendon reflexes, and sensory functions. The strength of all major muscle groups of upper and lower extremities is evaluated semiquantitatively and classified as 0, 25%, 50%, 75%, and 100% of weakness, resulting in scores of 0 to 4, respectively. Activity of tendon reflexes including the biceps, triceps, brachioradialis, patellar, and achilles reflexes are evaluated and categorized as normal, decreased, or absent, with the corresponding scores being 0, 1, and 2. Eventually, touch, pricking pain, vibration, and joint position on great toe and index finger are categorized as normal, decreased, or absent using scores of 0, 1, and 2, respectively.
-glutamyl transferase, prothrombin, thyreotropin-stimulating hormone, erythrocyte folate, immunoglobulin G, carbamide, M-component, and amylase were measured with standard laboratory techniques. Galactose elimination capacity (GEC)13 was determined at the time of the study except for a few patients. Eventually, all patients were evaluated by a trained neurologist according to a neuropathy symptom score14 and a neurological disability score.15 The neuropathy symptom score includes symptoms of motor, sensory, and autonomic disturbances. Motor symptoms are complaints about weakness of proximal and distal muscle groups of upper and lower extremities. In addition, complaints of facial and eye muscle palsy and difficulties in chewing and swallowing are noted. Sensory symptoms include difficulties in identifying objects with hand and in the mouth, unsteadiness during walking, numbness or paresthesia, and pain of neuropathic origin. Autonomic symptoms are postural fainting, male impotence, loss of urinary control, and nightly diarrhea. The neurological disability score is obtained from the neurological examination and includes scores of muscle strength, tendon reflexes, and sensory functions. The strength of all major muscle groups of upper and lower extremities is evaluated semiquantitatively and classified as 0, 25%, 50%, 75%, and 100% of weakness, resulting in scores of 0 to 4, respectively. Activity of tendon reflexes including the biceps, triceps, brachioradialis, patellar, and achilles reflexes are evaluated and categorized as normal, decreased, or absent, with the corresponding scores being 0, 1, and 2. Eventually, touch, pricking pain, vibration, and joint position on great toe and index finger are categorized as normal, decreased, or absent using scores of 0, 1, and 2, respectively.
Electrophysiological Studies and Quantitative Sensory Examination Tests. Nerve conduction studies were performed with standard surface stimulation and recording techniques using an electromyograph (DANTEC Counterpoint; Skovlunde, Denmark) with standard filter settings.16 Motor nerve conduction velocity (MNCV) and amplitude of the compound muscle action potential (MAP) were measured from the dominant median nerve and the nondominant peroneal nerve. Sensory nerve conduction velocity (SNCV) and amplitude of the sensory nerve action potential (SAP) were measured from the nondominant sural nerve and the dominant median nerve. For MNCV and MAP, Z-scores were calculated from values of healthy volunteers obtained with similar techniques.16 For SNCV and SAP, values previously determined in age-matched healthy controls were adopted.
Vibratory perception thresholds were determined at pulp of the dominant index finger and at nondominant dorsum of the great toe using forced choice techniques (Case IV; WR Medical Electronics Co., Stillwater, MN). Cooling perception thresholds were assessed at dorsum of the dominant hand and nondominant foot. The thresholds were obtained with the 4, 2, and 1 stepping algorithm.17 The perception thresholds for each patient were compared with the corresponding percentiles of a large group of healthy subjects.
Isokinetic Muscle Testing. The maximal isokinetic muscle strength (peak torque) of flexion and extension at ankle, hip, knee, elbow, and wrist, as well as abduction and adduction at shoulder, was evaluated with an isokinetic dynamometer (Lido Active Multijoint II; Loredan Biomedical, Inc., West Sacramento, CA). The nondominant leg and the dominant arm were tested with standardized procedures as described elsewhere. 18,19 After a presession, the subjects were instructed to push and pull as hard and fast as possible through the full available range of motion. Every test included eight reciprocal trials with a 10-second rest period between each trial. To exclude submaximal performance, data were accepted if the coefficient of variation for torque values was <10%. In experiments in which the coefficient of variation was greater than 10%, the subject was retested once. If the coefficient of variation still was greater than 10% at the second test, data were excluded if no out-layer torque curve could be identified.
For the ankle, knee, and wrist tests, subjects were positioned as described previously.19 At the elbow test, subjects were positioned in a semisupine position, and the axis of the dynamometer was aligned with an axis perpendicular to the lateral epicondylus. At the hip test, subjects were supine, and the axis of the dynamometer was aligned with an axis perpendicular to the major trochanter. At the shoulder test, subjects were lying on their nondominant side, and the axis of the dynamometer was aligned with an axis perpendicular to the acromion.
Nutritional Status. All patients were examined by a trained dietician. Triceps skinfold thickness was measured with a skin caliper.20 The values were compared with those of a large group of healthy subjects in the literature.21 The mid-arm circumference (MAC) was measured, and the mid-arm muscle area was calculated from the triceps skinfold thickness as described by Frisancho.22 Lean body mass (LBM) was determined from two 24-hour urinary creatinine excretions (LBM = 24-hour creatinine (in mmol) × 3.29 + 7.38).23 Results are percentages of the expected values using formulas provided by Shizgal.24 Eventually, the total lifetime dose of ethanol was estimated on the basis of an interview.
Calculations and Statistical Analysis. For each patient, predicted values for strength of knee and ankle flexors and extensors were calculated by the use of multiple regression analysis including the variables of age, height, and weight for each sex. Hereby the actual muscle strength of each patient was expressed as a percentage of the predicted strength. To quantify the degree of neuropathy, an individual neuropathy rank sum score (NRSS) was calculated. The NRSS was a summation of the rank scores from the neuropathy symptom score, neurological disability score, sensory perception thresholds, conduction velocities, and amplitudes of motor and sensory nerves. Scores of symptoms and signs of muscular weakness were excluded from the total neuropathy symptom and neurological disability scores. The sensory perception thresholds were ranked according to the sum of the two percentiles obtained from the hand and foot. The conduction velocities and amplitudes were ranked for each of the four nerves investigated, and the mean sum score of the four conduction studies was included in the total rank sum.
The primary study parameters were peak torque of flexion and extension at the ankle, knee, hip, elbow, and wrist and at shoulder abduction and adduction. To test the statistical significance of muscular strength differences between the cirrhotic patients and control subjects, unpaired t tests were applied. The relationships between the muscle strength and the measures of nutrition and neuropathy or laboratory findings were estimated with linear regression analysis. Eventually, multiple linear regression analysis was applied to disentangle the effect on muscle strength of neuropathy, stage of liver disease, duration of alcohol abstinence, and malnutrition.
RESULTS
The cirrhotic patients, aged 51 ± 4.6 years (average ± SD), had a body weight of 66 ± 14.6 kg and a height of 168 ± 8.5 cm. The control subjects, aged 53 ± 9.6 years, had a body weight of 68 ± 9.5 kg and a height of 170 ± 7.6 cm. At the initial admission for medical care, 19 patients had ascites, and 10 patients had edema, whereas 3 patients had neither edema nor ascites. Seven and 10 patients had a history of hematemesis and encephalopathy, respectively.
At the day of testing the median Child-Pugh score was 7 (range, 5-12). Seven patients had ascites. Thirteen were group A patients, 9 were group B patients, and 2 were group C patients. At the clinical examination, 11 patients had symptoms of muscle weakness, and 9 had sensory symptoms, primarily paresthesias. The interval between last alcohol intake and the time of study was 90 days (range, 5-960 days). The amount of alcohol ingested was 67 g/d (range, 1.4-140 g/d) during a period of 18.5 years (range, 5-40 years), resulting in an estimated total lifetime alcohol consumption of 460 kg (range, 5-1,073 kg). In 4 patients, a reliable history for alcohol consumption could not be obtained. The clinical examination resulted in a median neurological disability score of 13 (range, 0-30). Eighteen patients obtained scores for muscle weakness and 18 for abnormalities of tendon reflexes and/or sensory function. The Mini Mental State Examination resulted in a median score of 29 (range, 27-30). No patient was excluded from the study because of a low Mini Mental State Examination score.
The patients had an LBM of 35.6 kg (range, 23.0-52.0 kg), corresponding to 79% (range, 48%-109%) of the expected value. In 2 patients, the LBM could not be determined because 1 subject refused 24-hour urine collection and, in one case, the creatinine determination failed. The body mass index for all patients was 21.2 (range, 15.2-36.1). More detailed information about nutritional status including mid-arm muscle area and MAC is given for each sex in table 1. The median GEC was 1.4 mmol/min (range, 0.85-2.54 mmol/min). Laboratory findings for the patients are shown in table 2. In all patients, the methyl malonate level was within normal values. Furthermore, no patient had erythrocyte folate values below the lower limit, and no patient had an M-component. Sixteen patients had lowered serum albumin levels.
| View This Table | table 1. Clinical Data for Cirrhotic Patients |
| View This Table | table 2. Laboratory Findings for Cirrhotic Patients |
Abnormal thresholds of vibratory and cooling perception thresholds at foot as well as hand were found in 8 and 5 patients, respectively. The MNCV and MAP of the median nerve were 51.2 m/s (range, 39.6-56.3 m/s) and 6.0 mV (range, 1.8-11.0 mV), respectively. For the peroneal nerve, the MNCV was 39.5 m/s (range, 29.6-46.7 m/s), and the MAP was 3.2 mV (range, 0.3-6.5 mV). In 4 patients, nerve conduction studies of the peroneal nerve could not be performed because of atrophy of the extensor digitorum brevis muscle. SNCV and SAP of the median nerve were 50.5 m/s (range, 36.9-56.4 m/s) and 9.3 µV (range, 2.9-31.0 µV), respectively. SNCV of the sural nerve was 42.3 m/s (range, 38.0-50.0 m/s), and the SAP was 4.0 µV (range, 0.8-9.6 µV). In 10 patients, no action potential could be obtained from the sural nerve.
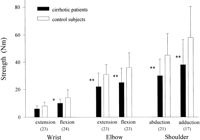
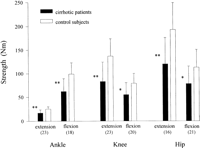
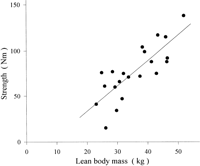
The isokinetic strength of all evaluated muscle groups was reduced in the patients compared with their matched control subjects (Figs. 1 and 2). For all muscle groups, a significant correlation between muscle strength and LBM was found (table 3). In Fig. 3 the relationship between LBM and the maximal strength of knee extension is shown. In patients with symptomatic muscle weakness, the strength of knee extension was 54% ± 22% of the expected value compared with a value of 71% ± 17% in asymptomatic patients (P < .05).
|
Fig. 1. Isokinetic muscle strength of the upper extremity for the cirrhotic patients and the matched healthy controls. Numbers of patients are given in parentheses. *P < .05; and **P < .01 |
|
Fig. 2. Isokinetic muscle strength of the lower extremity for the cirrhotic patients and the matched healthy controls. Numbers of patients are given in parentheses. *P < .01; and **P < .001. |
| View This table | table 3. Correlation Coefficient Between LBM (in kg) and Combined Muscle Strength (in Nm) at Ankle, Knee, Hip, Shoulder, Elbow, and Wrist |
|
Fig. 3. Isokinetic muscle strength of knee extension in relation to LBM for the cirrhotic patients (r2 = .62; P < .0005). |
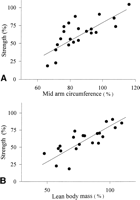
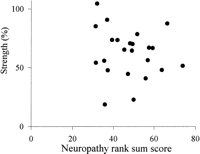
The muscle strength was related to the nutritional status. For the strength at the knee, significant correlations were found with MAC as well as with LBM (Fig. 4). Significant correlations were also found between the strength at the ankle and the LBM (r2 = .40; P < .005) and MAC (r2 = .52; P < .0005). In contrast, no correlation could be established between the GEC or the Child-Pugh score and the strength at the knee or the ankle. None of the laboratory findings given in table 2 was related to the strength at the knee. A significant positive correlation was found between the strength at the knee and the interval between last alcohol intake and the day of testing (interval between last alcohol intake and the day of testing) (r2 = .35; P < .003), whereas no correlation was observed at the ankle (r2 = .02; NS). Interval between last alcohol intake and the day of testing was also related to the LBM (r2 = .20; P < .04). No relationship could be established between the severity of neuropathy expressed as the NRSS and the strength at the knee (Fig. 5), whereas a weak correlation was present for the ankle (r2 = .18; P < .05). Multiple linear regression analysis including LBM, GEC, Child-Pugh score, interval between last alcohol intake and the day of testing, and NRSS as independent variables resulted in a significant effect only of the LBM at the knee (r2 = .62; P < .0001) as well as at the ankle (r2 = .40; P < .01). No relation was found between the LBM and the NRSS (r2 = .10; NS). Total lifetime alcohol consumption also did not relate to strength at the knee (r2 = 0; NS) or at the ankle (r2 = 0; NS).
|
Fig. 4. Averaged isokinetic muscle strength of knee extension and flexion expressed as a percentage of the predicted strength in relation to the (A) MAC, given as a percentage of the reference value (r2 = .56; P < .0001), and to the (B) LBM, expressed as a percentage of the predicted LBM (r2 = .62; P < .00005), for the cirrhotic patients. |
|
Fig. 5. Averaged isokinetic muscle strength of knee extension and flexion (r2 = .02; NS) expressed as a percentage of the predicted strength in relation to the NRSS for the cirrhotic patients. |
DISCUSSION
Muscle wasting is a well-known complication of liver cirrhosis in patients referred for medical care. Studies of motor performance and functional disability in patients with alcoholic liver cirrhosis are few. This is the first quantitative study of motor function in alcohol-induced cirrhotic patients. The study includes the relation of muscle strength to nutritional status, degree of liver disease, duration of alcohol abstinence, and nerve function. The main finding is a substantial impairment of the strength of all muscle groups in patients with alcohol-induced liver cirrhosis. The severity of weakness closely relates to malnutrition, whereas severity of peripheral neuropathy and liver disease and interval between alcohol intake and testing are not related to the muscle strength.
Population-based studies on elderly people have shown a close relation between muscle strength and functional ability,25 emphasizing the relevance of quantitative assessment of motor function. In the present study, the patients had considerable muscle weakness, which clearly impaired daily activities. Isokinetic dynamometry enables sensitive monitoring of motor deficits and of the effect of medical treament, including liver transplantation. In healthy subjects, muscle strength varies in relation to weight, height, age, and sex.26 Other factors such as occupational status and physical activity have a significant impact on muscle strength, although the relationship is rather complex. Manual work results in increased strength in younger subjects but decreased strength in older subjects.27 Therefore, to evaluate quantitatively the strength of a patient, incorporation of weight, height, age, and sex is necessary.
In cirrhotic patients, quantitative estimation of total muscle mass is difficult because of disturbed body composition with fluid retention. LBM and MAC more closely reflect the nutritional status compared with body weight and serum protein levels. 28,29 Recently, it has been reported that an estimation of total body muscle mass based on 24-hour creatinine excretion in cirrhotic patients resulted in falsely low values in patients with renal dysfunction caused by extrarenal excretion and recycling of creatinine to creatine.30 Nevertheless, in our study, a close correlation was found between strength of all muscle groups and the LBM estimated on the basis of renal creatinine excretion (table 3 and Fig. 3), whereas neither laboratory findings nor the stage of liver disease was related to the muscle strength. Also the LBM given as a percentage of the expected value was closely related to the muscle strength expressed as a percentage of the expected value (Fig. 4). The expected muscle strength of the individual patient was calculated on the basis of body weight, height, and sex. If a patient has loss of muscle mass without retention of fluid, this will result in a decrease of body weight and a lower predicted strength value. Consequently, this adjustment could result in normal relative strength. However, some of the cirrhotic patients had fluid retention; therefore, the decrease in relative muscle strength could partly be a consequence of overestimation of the predicted strength caused by alterations in body composition.
In addition to loss of muscle mass, motor dysfunction in cirrhotic patients could also be caused by qualitative impairment of the muscles. This can be evaluated by quantitative assessment of motor performance in relation to LBM enabling the calculation of intrinsic muscle strength (force production per unit muscle mass). In our study, LBM was not determined in the control subjects, and it is not clear whether the observed motor dysfunction is only caused by muscle wasting or by additional biochemical alterations of muscle function. A qualitative impairment can in fact be suspected because even the well-nourished patients had some muscle impairment, i.e., a strength of <100% of the expected value (Fig. 4).
Ethanol can induce changes in muscle function by inhibition of muscle membrane channels and pumps, as well as disturbances of protein synthesis and mitochondrial function.31 In our study, the interval between last alcohol consumption and testing was correlated inversely with the muscle strength at the knee, suggesting a direct effect of ethanol per se. However, including all relevant variables, only the nutritional status was correlated with the muscle strength. Thus, the relationship between the interval and muscle strength could be a consequence of the more recent insufficient nutritional intake during heavy alcohol drinking, resulting in a more malnourished state at the day of study. Decreased muscular content of energy-rich phosphagens observed in cirrhotic patients could contribute to impaired motor function.4 Also cirrhotic patients are insulin insensitive with concomitant abnormal glycogen deposition in striated muscles.32 In addition, accelerated protein degradation and a decreased content of magnesium in striated muscles have been reported. 3,5 All these abnormalities can contribute to the muscle impairment. Further studies are needed, however, to assess their exact influence on motor function.
In this study, the motor conduction velocities were more impaired than the amplitudes. Because proximal and distal parts of the nerves are affected equally, the impaired conduction velocity most likely is caused by metabolic impairment of the axolemma caused by collateral shunting and hepatocellular damage. 33,34 It is noteworthy that, in the majority of patients in this study, the amplitudes of the motor nerves were considerably less impaired than the conduction velocities. Considering the severity of weakness, the amplitudes were well preserved, which is in accordance with findings of other studies.35 The well-preserved amplitudes suggest that loss of muscle strength is not primarily caused by axonal degeneration and neurogenic muscle atrophy. This is further supported by the absence of a correlation between the severity of neuropathy and the strength at the knee (Fig. 5). This conclusion is in accordance with a recent study by Estruch et al.36 In 250 alcoholics, nearly 50% had myopathy defined as decreased muscle strength of shoulder abduction, whereas <20% had electrophysiological signs of peripheral neuropathy.
The prevalence of neuropathy depends on the definition of the condition. No single clinical or electrophysiological variable reliably reflects the degree of peripheral nerve damage in a patient partly because nerve pathology is widely spread in the peripheral nervous system. To allow a mutual and reliable grading of the severity of neuropathy, we calculated a NRSS for each patient based on the scores from the clinical examination, the quantitative sensory testing, and the electrophysiological studies. This NRSS has been found to be useful in other studies of motor function.19 It is noteworthy that this NRSS was not closely related to motor function in cirrhotic patients. Neurogenic atrophy in axonal polyneuropathies is located distally in the extremities. Also, the present finding of generalized weakness favors a metabolic muscle dysfunction. It is unlikely that the slight changes of peripheral nerve function found in the present study play an important role in the development of the severe muscle weakness of cirrhotic patients.
In conclusion, patients with alcoholic liver cirrhosis have substantial generalized weakness of both proximal and distal muscle groups. The weakness is related to malnutrition but not to degree of liver disease, duration of alcohol abstinence, total life dose of alcohol, or peripheral neuropathy. Further studies are warranted to assess whether muscles from cirrhotic patients are weak because of factors other than loss of mass and to decide whether hyperalimentation can improve motor function.
Footnotes
Abbreviations: GEC, galactose elimination capacity; MNCV, motor nerve conduction velocity; MAP, motor nerve action potential; SNCV, sensory nerve conduction velocity; SAP, sensory nerve action potential; MAC, mid-arm circumference; LBM, lean body mass; NRSS, neuropathy rank sum score.
Received June 13, 1997; accepted January 6, 1998.
Address reprint requests to: Henning Andersen, M.D., Department of Neurology, Aarhus University Hospital, Nørrebrogade 44, 8000 Aarhus C, Denmark. Fax: 45-8949-3300.




