Hepatology, February 1999, p. 328-333, Vol. 29, No. 2
Association of Diabetes Mellitus and Chronic Hepatitis C Virus Infection
Andrew L. Mason1,
From the 1Section of Gastroenterology and Hepatology, Alton Ochsner Medical Institutions, New Orleans, LA; 2Division of Gastroenterology, Hepatology, and Nutrition, University of Florida, Gainesville, FL; 3Department of Medicine, Cambridge University School of Clinical Medicine, England; 4Section of Endocrinology, Alton Ochsner Medical Institutions, New Orleans, LA; 5Division of Digestive Diseases and Nutrition, National Institute of Diabetes, and Digestive and Kidney Diseases, Bethesda, MD; and 6Department of Pathology and Laboratory Medicine, University of Florida, Gainesville, FL.
ABSTRACT
While patients with liver disease are known to have a higher prevalence of glucose intolerance, preliminary studies suggest that Hepatitis C virus (HCV) infection may be an additional risk factor for the development of diabetes mellitus. To further study the correlation of HCV infection and diabetes, we performed a retrospective analysis of 1,117 patients with chronic viral hepatitis and analyzed whether age, sex, race, Hepatitis B virus (HBV) infection, HCV infection, and cirrhosis were independently associated with diabetes. In addition, a case-control study was conducted to determine the seroprevalence of HCV infection in a cohort of 594 diabetics and 377 clinic patients assessed for thyroid disease. In the former study after the exclusion of patients with conditions predisposing to hyperglycemia, diabetes was observed in 21% of HCV-infected patients compared with 12% of HBV-infected subjects (P = .0004). Multivariate analysis revealed that HCV infection (P = .02) and age (P = .01) were independent predictors of diabetes. In the diabetes cohort, 4.2% of patients were found to be infected with HCV compared with 1.6% of control patients (P = .02). HCV genotype 2a was observed in 29% of HCV-RNA-positive diabetic patients versus 3% of local HCV-infected controls (P < .005). In conclusion, the data suggest a relatively strong association between HCV infection and diabetes, because diabetics have an increased frequency of HCV infection, particularly with genotype 2a. Furthermore, it is possible that HCV infection may serve as an additional risk factor for the development of diabetes, beyond that attributable to chronic liver disease alone. (HEPATOLOGY 1999;29:328-333.)
INTRODUCTION
Individuals with type II diabetes have an increased prevalence of cirrhosis, and a proportion of patients with acute and chronic liver disease develop diabetes mellitus.1,2 Also, patients with various forms of liver disease can be predisposed to impaired glucose tolerance because of corticosteroid and hydrochlorthiazide therapy or hemochromatosis.1,3 In addition to these known risk factors, there is now emerging epidemiological data to suggest that Hepatitis C virus (HCV) infection may also contribute to the development of diabetes. For example, glucose intolerance is observed more often in patients with HCV infection compared with controls with liver disease,4-7 and the frequency of HCV infection in European populations with type II diabetes has been reported to be higher than expected compared with the general population.8-10 While these investigations suggest an epidemiological association between HCV infection and type II diabetes, no large, controlled studies have been performed to support this conclusion.
To establish a potential relationship between HCV infection and diabetes, we performed three studies: 1) a retrospective cross-sectional study to determine the prevalence of diabetes in patients with HCV infection compared with those with chronic Hepatitis B virus (HBV) infection; 2) a seroprevalence study of anti-HCV antibody in a cohort of diabetics and a population of patients undergoing thyroid evaluation to determine the prevalence of HCV infection in diabetics and a representative outpatient control group; and 3) an HCV genotype study of diabetic and nondiabetic patients with HCV infection, because certain HCV genotypes have previously been shown to be associated with extrahepatic manifestations of disease.11,12
PATIENTS AND METHODS
Chronic Viral Hepatitis Cross-sectional Study. Available records from all outpatients referred to the St. Louis Veterans Administration Medical Center (n = 517), from 1978 to April 1994, or to the Ochsner Clinic, New Orleans, LA (n =600), from 1988 to June 1995, for evaluation of chronic viral hepatitis were abstracted. All chronic viral hepatitis patients with adequate documentation of abnormal serum aminotransferases for greater than 6 months, viral hepatitis serology, and endocrine assessment were included in the database. A diagnosis of HBV infection was made if patients had evidence of Hepatitis B surface antigen, with or without Hepatitis B e antigen or HBV DNA. HCV infection was diagnosed if patients were seropositive for anti-HCV, and confirmational testing was performed by either radioimmunoblot assay or HCV-RNA determination if the diagnosis was in doubt. Patients with a diagnosis of non-A, non-B hepatitis before 1989 who were subsequently found to have HCV infection were also included in the study.
Patients were assigned a diagnosis of diabetes mellitus if there was documented use of oral hypoglycemic medication or insulin; random glucose in excess of 200 mg/dL, or fasting glucose greater than 140 mg/dL on two occasions; or primary management for the treatment of diabetes.13 Liver biopsy data was available from 574 patients, and a diagnosis of cirrhosis was either established by histology (n = 207), or a presumptive diagnosis was made when patients developed ascites, hematologic evidence of hypersplenism, or a marked coagulopathy that contraindicated a liver biopsy (n= 35). Records were also evaluated for age, sex, and race. Patients were excluded from the final analysis if they had conditions that may predispose to hyperglycemia (n = 201), such as hemochromatosis, chronic pancreatitis, carcinoma of the pancreas, total parenteral nutrition, and corticosteroid or hydrochlorthiazide therapy.
Diabetes Case-Control Study. Consecutively collected serum samples were obtained from the pathology laboratory at Ochsner Clinic, New Orleans, LA, from 594 patients undergoing glycosylated hemoglobin estimation and 377 patients referred for radionucleotide thyroid scan. All patients were attending the main campus and peripheral affiliated clinics in the New Orleans and Baton Rouge regions; the former group was comprised of diabetics, and the latter patients were nondiabetic. Each sample was tested for anti-HCV (HCV EIA II Abbott Laboratories, Abbott Park, IL) without knowledge of the patients’ serological or endocrine status. The records of each diabetic patient were subsequently assessed for age, sex, race, and serum aminotransferases, which were categorized as either always normal, intermittently abnormal, or always abnormal.
The frequency of HCV genotypes was assessed in all reproducibly positive samples and compared with the distribution of genotypes of 95 HCV-infected patients attending the hepatology clinic. In addition, the serum samples from the HCV-infected diabetics were assessed for autoantibodies to insulin by radioimmunoassay, islet cell antigens by indirect immunofluorescence, and glutamate decarboxylase by a depletion enzyme-linked immunosorbent assay, as previously described.14-16
Records from each HCV antibody-positive diabetic were reviewed for the date of diagnosis of diabetes, type of diabetes mellitus, and dates of possible exposure to HCV infection or onset of hepatitis when known. All patients over the age of 40 presenting with diabetes and those without an insulin requirement were assigned a diagnosis of type II diabetes. Subjects who had a history of diabetic ketoacidosis or those presenting below the age of 30 with a clinical requirement for insulin were assigned a diagnosis of insulin-dependent or type I diabetes. The risk factors for HCV infection that were analyzed included intravenous drug abuse, blood or blood-product transfusion, major surgery, hemodialysis, household contact, sexual exposure, and tattoos. Patients were then classified into three categories: onset of diabetes mellitus before recorded risk factors for exposure to HCV infection; onset of diabetes occurring after risk of exposure to HCV infection; and an indeterminate onset of HCV infection having either no discernible risk factors, or risk factors both before and after the onset of diabetes. These studies were approved by the Ochsner Medical Foundation Clinical Investigations Committee.
HCV Genotyping. We used the nomenclature for HCV genotypes proposed by Simmonds et al.17 HCV genotypes were determined by restriction fragment length polymorphism of the nested polymerase chain reaction product using primers derived from the HCV 5′ untranslated region.18,19 Briefly, the 5′ untranslated region was reverse-transcribed and amplified by nested polymerase chain reaction using outer primers (antisense: 5′-TCATGGTGCACGGTCTACGAGACCT-3′, sense: 5′-CTGTGAGGAACTACTGTCTT-3′) and inner primers (antisense: 5′-CACTCGCAAG CACTATCAGGCAGT-3′; sense: 5′-TCACGCAGAAAGCGTCTAG-3′) as described previously.18,19 The amplicons were then digested by two sets of restriction enzymes, Hae III/Rsa I and Mva I/Hinf I, to differentiate HCV into various major genotypes (types 1-6). Subtypes la/c and lb were further differentiated by restriction with the enzyme, BstU I. Subtypes 2a/c and 2b, as well as subtypes 3a and 3b, were further differentiated by digestion with ScrFI.
Statistical Analysis. Univariate methods included a t test to compare means of groups for continuous variables, and for categorical variables, ![]() 2 analysis was used unless Fisher’s exact test was required for frequency tables when greater than 20% of the expected values were less than 5. The Bonferroni adjustment was used to correct the interpretation of significance for multiple comparisons. Following the removal of patients with conditions predisposing to hyperglycemia from the chronic viral hepatitis cohort, the categorical variables of sex, race, virological diagnosis, and histological diagnosis, as well as the continuous variable of age, were assessed in a multiple logistic regression model using the null hypothesis that their coefficients were statistically not different from zero. The multiple logistic regression models were constructed to process data by both forward selection and backward elimination, and were constructed to test the interaction of independent variables.
2 analysis was used unless Fisher’s exact test was required for frequency tables when greater than 20% of the expected values were less than 5. The Bonferroni adjustment was used to correct the interpretation of significance for multiple comparisons. Following the removal of patients with conditions predisposing to hyperglycemia from the chronic viral hepatitis cohort, the categorical variables of sex, race, virological diagnosis, and histological diagnosis, as well as the continuous variable of age, were assessed in a multiple logistic regression model using the null hypothesis that their coefficients were statistically not different from zero. The multiple logistic regression models were constructed to process data by both forward selection and backward elimination, and were constructed to test the interaction of independent variables.
RESULTS
Factors Associated With Diabetes in the Chronic Viral Hepatitis Cross-sectional Study. Diabetes was detected in 24% of HCV-infected individuals as compared with 13% of those with HBV infection alone (P < .0001; 95% CI: 1.4-2.4). However, other significant differences were observed between patients with HBV infection and HCV infection in other characteristics such as age, sex, race, and the number of diabetics excluded from the final analysis (table 1). For example, the HCV cohort had a higher proportion of patients over the age of 61, women, subjects of European descent, and fewer patients of African or Asian descent. A total of 201 patients were excluded for criteria predisposing to hyperglycemia (table 1). The majority of the patients excluded were on corticosteroid immunosuppression following orthotopic liver transplantation. The proportion of patients with exclusion criteria were similar in the groups with HBV and HCV infection, but 34% of the excluded patients in the HCV cohort were diabetic, compared with 18% of the HBV-infected population (P = .015; 95% CI: 1.1-3.0).
| table 1. Characteristics of All Patients in the Chronic Viral Hepatitis Cohort |
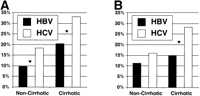
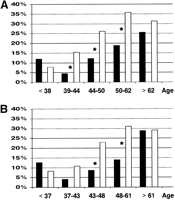
After the removal of patients with conditions causing hyperglycemia (table 2), diabetes was observed in 21% of patients with HCV infection compared with 12% of subjects infected with HBV (P = .0004; 95% CI: 1.3-2.4). Patients with cirrhosis were more likely to have glucose intolerance compared with those without (23% vs. 14%; P < .02), but sex and race had little impact on the diagnosis of diabetes (table 2). For patients with cirrhosis, the prevalence of diabetes was significantly greater in those with HCV infection compared with subjects infected with HBV (Fig. 1A and 1B). In the noncirrhotic cohort, however, the frequency of diabetes was only significantly greater in those with HCV infection when the entire cohort was entered into the analysis (Fig. 1A). The effect of aging on the development of diabetes was assessed by stratifying the chronic viral hepatitis cohort by quintiles for age. In this analysis, HCV infection was associated with an increased prevalence of diabetes in all but the youngest quintile (Fig. 2A and 2B). Antiviral therapy had little impact on the prevalence of diabetes, because 18% of those with a diagnosis of diabetes and 17% without had a recorded use of interferon alfa.
| table 2. Analysis of Chronic Viral Hepatitis Cohort for Variables Associated With Diabetic Criteria After Exclusion of Patients With Conditions Predisposing to Hyperglycemia |
| Fig. 1. Frequency of diabetes in HBV-infected and HCV-infected patients with absence of cirrhosis by liver biopsy compared with those with clinical or liver biopsy evidence of cirrhosis. (A) The entire cohort (HBV vs. HCV, noncirrhotic: 9.7% vs. 18.4%, P = .02; cirrhotic: 20.5% vs. 33.1%, P = .04 ); (B) After excluding subjects with conditions predisposing to diabetes (HBV vs. HCV, noncirrhotic: 16% v. 11%, P = .26; cirrhotic: 28% vs. 15%; P = .04 ). *P < .05 for differences in frequency of diabetes in HBV cohort vs. HCV. |
|
Fig. 2. Frequency distribution of diabetes in patients with either HBV infection or HCV infection stratified by quintiles of age. (A) The entire cohort (HBV vs. HCV from youngest to oldest quintile, 12% vs. 8%, P = .32; 5% vs. 15%, P = .03; 12% vs. 26%, P = .02; 19% vs. 36%, P = .01; 26% vs. 31%, P = .34 ). (B) After excluding subjects with conditions predisposing to diabetes (HBV vs. HCV from youngest to oldest quintile, 13% vs. 8%, P = .36; 4% vs. 11%, P = .14; 9% vs. 23%, P = .02; 14% vs. 31%, P = .01; 28% vs. 29%, P = .91 ). *P < .05 for differences in frequency of diabetes in HBV cohort vs. HCV. ( |
The multivariate analyses revealed that patient age and HCV infection were the only significant independent predictors for diabetes. The interaction of cirrhosis and diabetes was assessed in a second logistic regression for patients with available histological data, and no relationship was observed between these two variables. In the logistic regression, the age of the patient (P = .01) and HCV infection (P = .02) were associated with diabetes, and the relative odds for HCV-infected patients developing diabetes was calculated to be 2.1 (95% CI: 1.12-3.90) in this data set.
Characterization of HCV-Infected Patients in the Diabetes Case-Control Study. In the diabetic cohort, 25 of 596 samples were found to be reproducibly anti-HCV-positive compared with 6 of 377 samples derived from thyroid disease controls (4.2% vs. 1.6%; P = .02). With regard to liver function tests, consistently elevated serum aminotransferases were seen in 32% of the HCV-infected diabetics compared with 5% of those without infection (P < .0001) (table 3). Only 2 patients from the cohort of HCV-infected diabetics (8%) had type I diabetes, and of the 23 patients with HCV infection and type II diabetes, 12 (52%) had risk factors for the development of HCV before the onset of diabetes, and 11 (48%) had an indeterminate onset of HCV infection. None of the HCV-infected non-insulin-dependent diabetics had singular risk factors for HCV infection after the onset of diabetes. Autoantibodies were detected in 7 HCV-infected diabetics. Two patients had anti-glutamate decarboxylase (8%), 1 had anti-islet cell antigens (4%), and 5 had anti-insulin (20%) antibodies. Four patients in the latter group had type II diabetes, 1 had type I diabetes, and all were receiving insulin therapy.
| table 3. Characteristics of Diabetic Cohort Assessed by EIA II for Anti-HCV Status |
Analysis of the HCV-infected diabetic samples revealed different frequencies of HCV genotypes compared with local controls (table 4). For example, genotype 2a was detected in 6 of 21 diabetic patients versus 3 of 95 local HCV-infected patients (29% vs. 3%; P < .005; 95% CI: 3.0-27). In contrast, genotype 1a was found in 45% of the local population and was seen in 14% of the diabetic cohort (P < .05; 95% CI: 0.13-0.75).
| table 4. Prevalence of HCV Genotypes in HCV-RNA-Positive Samples From National and Local Subjects Compared With the Diabetic Cohort |
DISCUSSION
These studies provide epidemiological and virological data to link HCV infection and diabetes. In the liver disease cohort, diabetes was observed in 21% of patients with HCV infection, as compared with only 12% of HBV-infected patients. In the diabetes cohort, more than 20% of patients with consistently elevated serum aminotransferases had evidence of HCV infection. In the whole diabetic population studied, the prevalence of HCV infection (4.2%) was approximately 2.5 times greater than our outpatient control group. In marked contrast, the seroprevalence of Hepatitis B surface antigen in our diabetic patients (0.3%; data not shown) was comparable with the prevalence observed in our local blood donors and current estimates for chronic HBV infection reported for the United States.20 Taken together, these data suggest that HCV infection is more closely associated with diabetes than HBV infection, and this association cannot be attributed to chronic liver disease alone.
Our findings are in concordance with similar epidemiological studies from Europe and the Middle East. A striking observation from all the studies with chronic viral hepatitis cohorts of more than 300 patients, including our own, is the consistent finding that diabetes was observed in 24% to 26% of patients with HCV infection compared with 9% to 13% of patients with HBV infection and other liver disease controls.6,7,21 In the smaller studies with a greater proportion of patients with cirrhosis, the prevalence of diabetes was observed to be even higher in patients with HCV infection, ranging from 39% to 50%.4,5,7 In agreement, we also observed that cirrhosis increased the chances of glucose intolerance, because 33% of HCV-infected patients with cirrhosis had evidence of diabetes (Fig. 1A).
Age, cirrhosis, and HCV infection were found to be significant variables associated with diabetes by univariate analysis in our liver disease cohort (table 2). Because there were significant differences between the cohorts with HCV and HBV infection, no firm conclusions could be drawn from this analysis concerning the relative contribution of each variable to glucose intolerance. When patients were segregated by quintiles for age, an increased frequency of diabetes was observed in patients with HCV infection in all but the youngest age range (Fig. 2). The increased prevalence of diabetes in the HBV-infected individuals in the youngest quintile may be partially explained by the high frequency of Asians in this group (HBV = 13% vs. HCV = 1%), who contributed to the increased proportion of diabetics in this cohort.
The logistic regression analysis confirmed that age and HCV infection were independent predictors for diabetes mellitus. In support of this finding, Fraser et al. also documented that both HCV infection and increasing age were independent risk factors for diabetes, while cirrhosis had an insignificant role in their logistic regression analysis of a large cohort of patients with chronic viral hepatitis.5 Likewise, our multivariate analysis determined that cirrhosis was not an independent risk factor for diabetes in our population, even though cirrhosis is known to cause glucose intolerance.1 The diminished contribution of cirrhosis to the development of diabetes in our patients is illustrated by Fig. 1, in which the prevalence of diabetes was similar in HCV-infected individuals without cirrhosis compared with cirrhotic patients with HBV infection. Taken together, these findings suggest that HCV infection is a more important predictor of glucose intolerance than cirrhosis, and the combination of both factors further increases the risk of diabetes.
In the chronic hepatitis cross-sectional study, attempts were made to exclude patients with potentially confounding variables associated with diabetes. As a result, 34% of the HCV-infected patients excluded from the final analysis had diabetes compared with only 18% of those with HBV infection excluded for conditions predisposing to diabetes (table 1). However, there were other factors related to either liver disease or diabetes that were not satisfactorily addressed in this study. For example, data concerning increased body mass index and evidence of nonalcoholic steatohepatitis were not derived for the study, both of which are associated with type II diabetes.22 Another variable not addressed in this study was alcohol consumption on the assumption that the prevalence of alcohol abuse would be evenly distributed irrespective of viral diagnosis, and also because patients with evidence for pancreatitis were excluded from the final analysis. Of note, other investigators have reported that the prevalence of diabetes is lower in patients with alcohol-related liver disease alone as compared with those with chronic HCV infection.6 Although the specific effects of antiviral regimens were not completely addressed, it is unlikely that interferon alfa treatment had a substantial effect on the development of diabetes, because the frequency of diabetes was similar in the treated and nontreated patients.
This is the first case-control study known to the authors that assesses the anti-HCV seroprevalence in a diabetic population using an outpatient comparison group. Significant differences in the frequency of HCV infection were observed in our diabetic patients compared with those being assessed for thyroid disease (4.2% vs. 1.6%) as well as first-time blood donors (4.2% vs. 0.8%; data not shown). However, the latter group often does not provide adequate control data for a study of viral hepatitis, because blood donors are volunteers screened for exposure to blood products and behavior that increase the risk of developing viral hepatitis. In agreement with our findings, other studies have reported an increased seroprevalence of HCV infection, varying from 8% to 11%, in European diabetic populations in comparison with their local blood donors or the expected national frequency.8-10
The unusual distribution of HCV genotypes in our diabetic population merits further attention, even though the limited number of patients studied prevents definite conclusions. HCV genotypes 1a and 1b are found in approximately 70% of HCV-infected individuals in North America23 and were only demonstrated in 38% of our diabetic cohort (table 4). Furthermore, we observed a markedly increased frequency of genotype 2a in our diabetic cohort (29%) (table 4). This genotype was only found in 3% of our local population and in 4% of HCV-infected individuals in the United States.23 This may be a biologically significant finding, because preliminary reports suggest that HCV genotype 2a is preferentially associated with extrahepatic syndromes associated with HCV infection such as mixed cryoglobulinemia and benign monoclonal gammopathy.11,12 Further study of this interesting association between HCV gentoypes and diabetes is warranted.
In humans, there is now preliminary evidence to link other viruses, such as Coxsackie virus, with the development of type I diabetes.24 The P2-C protein of Coxsackie B virus shares regional amino acid homology with glutamate decarboxylase, an islet cell antigen, providing a possible mechanism for the induction of autoimmunity by viral molecular mimicry of host proteins.24 In regard to the latter, autoantibodies to islet cell antigens were rarely observed in our cohort of HCV-infected diabetics.
In conclusion, we have established an association between diabetes mellitus and HCV infection. It remains to be determined whether HCV infection leads to diabetes or vice versa. One could argue that patients with diabetes mellitus have an increased risk of exposure to HCV infection. However, the similar frequencies of HBV infection in our diabetic cohort and the local blood donors argues against diabetics having a significantly increased risk of exposure to hepatitis agents. Likewise, the significant abnormalities in distribution of HCV genotypes observed in our diabetic cohort are unlikely to be attributable to chance alone. Nevertheless, HCV infection cannot be considered to be a cause of diabetes without establishing a temporal relationship for the development of each disorder, and prospective studies are clearly needed to clarify these issues. In addition, demonstration of the specific endocrine abnormalities associated with HCV infection and improvement in glucose tolerance during antiviral therapy would strengthen the association of HCV infection and diabetes. These studies are warranted because it could lead to a different means of approaching the management of this common and serious endocrine disease.
Acknowledgment
The endocrine advice from Steven Giddings, M.D., and Janet McGill, M.D. (Washington University, St. Louis, MO), as well as Alan Burshell, M.D. (Alton Ochsner Medical Institutions, New Orleans, LA), was greatly appreciated while planning this study. The authors also thank Abe Wattar, M.D., Deborah Baudy, B.S., and Elizabeth Jones, B.A. (Alton Ochsner Medical Institutions, New Orleans, LA), for assistance with serology and HCV genotyping. In addition, they are indebted to Mary Kuhns, Ph.D. (Abbott Laboratories, North Chicago, IL), for providing serological reagents.
Abbreviations
HCV, Hepatitis C virus; HBV, Hepatitis B virus
Footnotes
Received March 31, 1998; accepted August 24, 1998.
Supported by research grants NATO (CRG 920697) Collaborative Research Grant (to A.L.M., G.J.M.A., and R.P.P.); a Hans Popper Scholar Award from the American Liver Foundation and a Glaxo Institute of Digestive Health Clinical Investigator Award (to J.Y.N.L.); and (RO1. HD19469-11) National Institutes of Health (to N.K.M.).
Dr. Lau’s current address is: Schering Plough Research Institute, Kenilworth, NJ.
Dr. Regenstein’s current address is: Department of Surgery, Tulane University Medical Center, New Orleans, LA.
Noel K. Maclaren’s current address is: Research Institute for Children, Harahan, LA.
Address reprint requests to: Dr. Andrew L. Mason, Section of Gastroenterology and Hepatology, Alton Ochsner Medical Institutions, 1520 Jefferson Highway, New Orleans, LA 70121. E-mail: amason@ochsner.org; fax: (504) 842-3792.
REFERENCES
Copyright © 1999 by the American Association for the Study of Liver Diseases.