Full-Length Complementary DNA of Hepatitis C Virus Genome From an Infectious Blood Sample
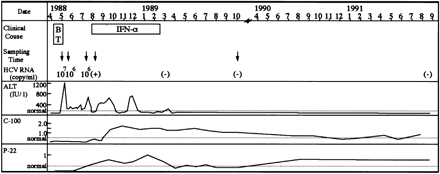
Fig. 1. Clinical characteristics of the recipient. The profiles of ALT values (IU/L) and anti-HCV antibody (C-100 and p-22) titers in the recipient from March 1988 to September 1991 are shown. Open squares indicate the periods of blood transfusion (BT) and IFN treatment (IFN-![]() ). HCV RNA titer was measured by use of competitive PCR.24 HCV RNA, whose titer was less than 103, was detected by using RT-nested PCR of the 5′ noncoding region.25 Arrows denote the times when plasma specimens were obtained for analysis of HCV RNA.
). HCV RNA titer was measured by use of competitive PCR.24 HCV RNA, whose titer was less than 103, was detected by using RT-nested PCR of the 5′ noncoding region.25 Arrows denote the times when plasma specimens were obtained for analysis of HCV RNA.