Hepatology, March 1999, p. 664-669, Vol. 29, No. 3
Cryptogenic Cirrhosis: Clinical Characterization and Risk Factors for Underlying Disease
Stephen H. Caldwell1,
From the 1Department of Internal Medicine, Division of Gastroenterology and Hepatology, and 2Department of Pathology, University of Virginia, Charlottesville, VA.
ABSTRACT
We characterized 70 consecutive patients with cryptogenic cirrhosis to assess major risks for liver disease. Each patient was reevaluated for past alcohol exposure, scored by the International Autoimmune Hepatitis (IAH) score and assessed for viral hepatitis risks and risks for nonalcoholic steatohepatitis (NASH). The results were compared with 50 consecutive NASH patients, 39 nonalcoholic patients age 50 and over with cirrhosis from hepatitis C, and 33 consecutive patients with cirrhosis caused by primary biliary cirrhosis (PBC). Among the cryptogenic group, 49 (70%) were female, and the mean age was 63 ± 11 years. Although ascites and variceal bleeding were common, almost one half lacked major signs of complicated portal hypertension. A history of Type 2 diabetes mellitus and/or obesity was present in 51 (73%). Nineteen (27%) patients had a history of blood transfusions antedating the diagnosis of cirrhosis. No clinical or histological features distinguished this group from the other patients, and 14 (74%) of these had a history of obesity and/or diabetes. Nineteen of the remaining nontransfused patients had indeterminant IAH scores but were histologically and biochemically indistinguishable from the others. Twelve of these (63%) also had a history of obesity and/or diabetes. Both diabetes and obesity were significantly more common in the cryptogenic cirrhotic patients compared with the cirrhotic patients with PBC or hepatitis C. In contrast, the prevalence of obesity and diabetes was similar to the NASH patients who were, on average, a decade younger. Although there is some diversity that indicates more than one cause, our findings suggest that NASH plays an under-recognized role in many patients with cryptogenic cirrhosis, most of whom are older, type 2 diabetic and obese females. (HEPATOLOGY 1999;29:664-669.)
INTRODUCTION
Cirrhosis is usually accepted as “cryptogenic” only after an extensive evaluation has excluded recognizable etiologies. The prevalence of cryptogenic cirrhosis ranges from 5% to 30% of cirrhotic patients in past series.1,2 Several explanations may be offered as possible underlying etiologies. These include occult alcohol abuse, occult viral (non-B, non-C) hepatitis, silent autoimmune hepatitis, or progression of nonalcoholic steatohepatitis (NASH).3 ![]() 1-Antitrypsin phenotype abnormalities, such as phenotype MZ, are sometimes present among patients with cryptogenic cirrhosis in the absence of frank deficiency, but heterozygous carriage of these phenotypes is usually thought to potentiate some other condition rather than to explain cirrhosis.4
1-Antitrypsin phenotype abnormalities, such as phenotype MZ, are sometimes present among patients with cryptogenic cirrhosis in the absence of frank deficiency, but heterozygous carriage of these phenotypes is usually thought to potentiate some other condition rather than to explain cirrhosis.4
The prevalence of clinically silent autoimmune hepatitis is not known; however, asymptomatic patients with autoimmune hepatitis and previously unrecognized cirrhosis have been described.5,6 Non-B, non-C hepatitis is thought to account for about 15% of posttransfusion hepatitis7 and may exist in a silent form for years.8 Obesity and non-insulin-dependent diabetes mellitus are the two most common conditions associated with NASH,9 which is frequently asymptomatic10and which can progress silently to cirrhosis with loss of definitive histological features.3,11-13 Our goal in the present study was to characterize our patients with cryptogenic cirrhosis with attention to major risk factors that might offer an explanation for their condition. After our initial analysis revealed a predominance of older females with NASH risk factors (obesity and diabetes), we compared the cryptogenic group with patients with NASH without cirrhosis and with two groups of other cirrhotic patients: one with cirrhosis caused by hepatitis C without prior alcoholism and one with cirrhosis caused by primary biliary cirrhosis (PBC).
PATIENTS AND METHODS
We reviewed all of the available clinical information on 102 patients with cryptogenic cirrhosis encountered over 3 years and confirmed the data (with particular attention to the alcohol history and other risk factors for liver disease) by follow-up interview of the patients and their families. The patients were ascertained from our Liver Disease Registry, which has been kept since 1994. The diagnosis of cryptogenic cirrhosis was made only after an exhaustive evaluation failed to define a specific etiology. Our registry is maintained by one of the authors (S.C.), and, at the time of this study, it contained approximately 1,200 patients with various liver diseases. Recorded data include the major hepatologic diagnoses, comorbid conditions, complications of portal hypertension if present, and major forms of treatment. Additional information was obtained from clinical charts, hospital records, the clinic and hospital laboratory databases, and personal or telephone follow-up.
The patients were included if sufficient data were available and if the diagnosis was confirmed on review of all available information. For the purposes of this study, we defined possibly significant alcohol exposure as consistent dailyconsumption of any amount of alcohol for over 1 year at any time in the patient’s life. Thirty-two of 102 patients were excluded. In 22 of these, there was incomplete clinical or laboratory data. In 10, careful review of the history or telephone follow-up revealed possibly significant past alcohol exposure. Sufficient data were available and confirmed in 70 patients, who thus constituted the study group.
The diagnosis of cirrhosis was made on the basis of compatible clinical and imaging findings in all patients and histological findings in 52 of 70 patients. Biopsy was not performed in 28 patients because of either patient or primary physician refusal, together with convincing clinical and laboratory data. Gender, age at diagnosis of cirrhosis, presenting symptomatology, potential occupational exposure to hepatotoxins, family history of liver disease, and family or personal history of autoimmune diseases were evaluated. Risk assessment for viral hepatitis including past exposure to intravenous drugs, blood transfusions, tattoos, other known percutaneous needle exposures, or high-risk sexual behavior were determined. None of the patients had any risks other than blood transfusions (which antedated the diagnosis of cirrhosis) in 19 patients.
All of the patients underwent extensive serological testing including hepatitis B and C screens (hepatitis B surface antibody, surface antigen, and anticore antibody, and hepatitis C enzyme-linked immunosorbent assay [Abbott Laboratories, Abbott Park, IL]), iron studies (ferritin, iron, iron binding capacity, and tissue assessment if questionable), ceruloplasmin levels, antinuclear antibody (ANA) titers, antimitochondrial antibody titers, and ![]() 1-antitrypsin level. Quantitative immunoglobulin levels (IgG, IgM, IgA) were performed in 52 patients.
1-antitrypsin level. Quantitative immunoglobulin levels (IgG, IgM, IgA) were performed in 52 patients. ![]() 1-Antitrypsin phenotyping was performed in 42 patients using isoelectric focusing (pH range, 4.0-5.0). Hepatitis G was tested for in 31 patients using a reverse-transcription polymerase chain reaction (Genelabs Technologies Inc., Redwood City, CA) as previously reported.14,15 HLA typing for Class I and II antigens was performed by serological assay in 25 patients. Anti-liver kidney microsomal antibody was measured in only 13 patients (all negative) but was not felt to be critical to the study because of its infrequency in our experience (unpublished observations) and in published reports from this country.16,17 Fasting serum lipid profiles were generally not available and thus were not analyzed.
1-Antitrypsin phenotyping was performed in 42 patients using isoelectric focusing (pH range, 4.0-5.0). Hepatitis G was tested for in 31 patients using a reverse-transcription polymerase chain reaction (Genelabs Technologies Inc., Redwood City, CA) as previously reported.14,15 HLA typing for Class I and II antigens was performed by serological assay in 25 patients. Anti-liver kidney microsomal antibody was measured in only 13 patients (all negative) but was not felt to be critical to the study because of its infrequency in our experience (unpublished observations) and in published reports from this country.16,17 Fasting serum lipid profiles were generally not available and thus were not analyzed.
An index of autoimmune hepatitis, the International Autoimmune Hepatitis (IAH) score, was calculated for each patient based on clinical and laboratory parameters as previously described.18 None of the patients received steroid therapy, and thus the IAH score was calculated using the Minimal Required Parameters, wherein a score of 10 to 15 is suggestive of autoimmune hepatitis, and a score of greater than 15 is considered definitive. Obesity was defined as a body mass index (BMI) greater than 31.1 for men and 32.3 for women (95th percentiles), rather than the lower levels of moderate obesity (overweight) at the 85th percentile (27.8 for men and 27.3 for women).19-21 BMI > 30 is almost always associated with increased body fat.21 Because cirrhosis is associated with changes in body mass and protein calorie malnutrition,22,23 we assessed the patients for a history of obesity by inquiring about the patients’ average adult weight before the diagnosis of cirrhosis. In addition, it is known that most adults gain weight through middle age and then lose weight with advancing years.19As has been previously described, BMI was thus calculated using the average adult weight reported by the patient and the patient’s height.24 The majority of historically obese patients remained obese at the time of their evaluation. Type 2 diabetes and obesity are thought to be independent risk factors for NASH,25and thus were considered separately and in combination. In all cases, diabetes mellitus (Type 2 in all of the cryptogenic patients) had been diagnosed by the primary care physician based on recurrent fasting hyperglycemia requiring either dietary management, oral hypoglycemics, or insulin therapy.
Among the 70 cryptogenic cirrhosis patients, observations were made between four major subsets based on risk assessment as follows: (1) history of a blood transfusion before the diagnosis of cirrhosis (none had other percutaneous exposure), regardless of the history of obesity/diabetes or the autoimmune score (N = 19); (2) elevated autoimmune score (![]() 10) without prior transfusion and regardless of the history of diabetes or obesity (N = 19); (3) history of obesity and/or diabetes mellitus without antecedent blood transfusions and with a low (<10) autoimmune score (N = 25); and (4) those with no identifiable risk factors (N = 7).
10) without prior transfusion and regardless of the history of diabetes or obesity (N = 19); (3) history of obesity and/or diabetes mellitus without antecedent blood transfusions and with a low (<10) autoimmune score (N = 25); and (4) those with no identifiable risk factors (N = 7).
Secondary comparisons were made between the entire group of cryptogenic cirrhosis patients and 50 consecutive patients with NASH encountered over the same period of time, 39 nonalcoholic patients with cirrhosis caused by hepatitis C selected for age ![]() 50 years, and 33 nonselected, consecutive patients with antimitochondrial antibody-positive PBC and cirrhosis on biopsy. The former were ascertained from 75 NASH patients then in the registry, among whom sufficient data were available in 50 patients. The hepatitis C patients were age selected to represent a group of nonalcoholic patients with chronic viral hepatitis and cirrhosis to compare the prevalence of diabetes and obesity in a similar-aged group of cirrhotic patients as the cryptogenic group. These patients were ascertained from over 500 hepatitis C patients (184 with cirrhosis) then in our registry and age matched to the extent that all were age 50 or older. The PBC patients were ascertained from approximately 70 PBC patients and chosen if they had positive antimitochondrial antibody (by immunofluorescence) and histological cirrhosis. There were 33 such patients—all with sufficient available data for inclusion.
50 years, and 33 nonselected, consecutive patients with antimitochondrial antibody-positive PBC and cirrhosis on biopsy. The former were ascertained from 75 NASH patients then in the registry, among whom sufficient data were available in 50 patients. The hepatitis C patients were age selected to represent a group of nonalcoholic patients with chronic viral hepatitis and cirrhosis to compare the prevalence of diabetes and obesity in a similar-aged group of cirrhotic patients as the cryptogenic group. These patients were ascertained from over 500 hepatitis C patients (184 with cirrhosis) then in our registry and age matched to the extent that all were age 50 or older. The PBC patients were ascertained from approximately 70 PBC patients and chosen if they had positive antimitochondrial antibody (by immunofluorescence) and histological cirrhosis. There were 33 such patients—all with sufficient available data for inclusion.
Histological Review.
Liver tissue was available for inspection on 41 of 52 of the cryptogenic patients who underwent biopsy (11 outside biopsies were not available). The specimens were assessed blindly according to the scheme recently described by Batts and Ludwig.26 After the initial blinded review, we specifically sought histological correlation with the clinical subset determined by the major risk-factor grouping for each patient to determine if there existed traces of prior active disease characteristics of a given group. Specifically, we sought evidence of residual steatohepatitis, plasma cell infiltration, or lymphoid aggregates that might suggest a primary etiology. The finding of steatohepatitis required the presence of necroinflammatory activity in the setting of fatty infiltration.27 The presence of Mallory bodies, seen in 6 specimens (see below), was not considered sufficient for the diagnosis of steatohepatitis.28
Statistical Analysis.
Comparisons were made using the Student t test for comparison of means and the ![]() 2 test with the Yates correction for comparison of proportions, performed on a personal computer (Minitab Statistical Software, Reading, MA). An odds ratio analysis was performed using SAS statistical software (Cary, NC). All computations are expressed as means ± SD.
2 test with the Yates correction for comparison of proportions, performed on a personal computer (Minitab Statistical Software, Reading, MA). An odds ratio analysis was performed using SAS statistical software (Cary, NC). All computations are expressed as means ± SD.
RESULTS
The 70 cryptogenic patients are summarized in Tables 1 and 2. The mean age was 63 ± 11 years (range, 39-89 years), and 49 patients (70%) were female. The age and sex distribution in the 32 excluded patients did not differ significantly from the study group (mean age, 64 ± 10 years; 72% female). The aminotransferase levels and alkaline phosphatase tended to be normal or mildly abnormal. Only 56% presented with major complications of portal hypertension (ascites, bleeding, or encephalopathy) (table 2). Twenty of 70 patients (29%) had a history of known, mild liver enzyme abnormalities 1 or more years before the diagnosis of cirrhosis, and in 8 (11%), this constituted the major presenting abnormality. Five patients reported possible occupational exposure to solvents. The solvents were identified in 2 patients: trichloroethylene in 1 and furniture refinishing solvents in the other.
| table 1. Demographic, Clinical, and Laboratory Parameters of 70 Patients With Cryptogenic Cirrhosis |
| table 2. Major Presenting Sign or Symptom in Cryptogenic Cirrhosis |
The ![]() 1-antitrypsin phenotype was abnormal in 14 of 42 patients (33%): 7 MS, 5 MZ, 1 MF, and 1 SS. However, none of the patients had biochemical or histological evidence of
1-antitrypsin phenotype was abnormal in 14 of 42 patients (33%): 7 MS, 5 MZ, 1 MF, and 1 SS. However, none of the patients had biochemical or histological evidence of ![]() 1-antitrypsin deficiency. Eleven patients (16%) had elevated ferritin levels. Iron saturation was greater than 50% in 5 patients, but none of these had sufficient iron stores evident (by staining or tissue quantitation) to indicate primary hemochromatosis. Genetic testing for hemochromatosis was not performed, and thus carriage of abnormal alleles, recently described in some patients with NASH,29 cannot be excluded. Three of 31 patients were reverse-transcription polymerase chain reaction-positive for hepatitis G RNA, but all 3 had received blood transfusions only after the diagnosis of cirrhosis. Regarding HLA haplotypes associated with autoimmune hepatitis,30 11 of 25 patients (44%) had HLA A1 (only 1 of these had an elevated autoimmune score), 6 of 25 (24%) had HLA B8, 3 of 25 (12%) had HLA DR3, and 2 of 25 (8%) had HLA DR4. Only 1 had the extended HLA A1-B8-DR3 haplotype.
1-antitrypsin deficiency. Eleven patients (16%) had elevated ferritin levels. Iron saturation was greater than 50% in 5 patients, but none of these had sufficient iron stores evident (by staining or tissue quantitation) to indicate primary hemochromatosis. Genetic testing for hemochromatosis was not performed, and thus carriage of abnormal alleles, recently described in some patients with NASH,29 cannot be excluded. Three of 31 patients were reverse-transcription polymerase chain reaction-positive for hepatitis G RNA, but all 3 had received blood transfusions only after the diagnosis of cirrhosis. Regarding HLA haplotypes associated with autoimmune hepatitis,30 11 of 25 patients (44%) had HLA A1 (only 1 of these had an elevated autoimmune score), 6 of 25 (24%) had HLA B8, 3 of 25 (12%) had HLA DR3, and 2 of 25 (8%) had HLA DR4. Only 1 had the extended HLA A1-B8-DR3 haplotype.
Laboratory parameters (table 1) were not significantly different between the risk subgroups. We observed a preponderance of females in the group with indeterminant autoimmune scores, although this may be artifactual as a result of the criteria used to define the score, which includes female sex as a positive value (+2). As in the entire study group, the majority of patients in the blood-transfusion group and in the indeterminant autoimmune group were either obese and/or diabetic. Eleven patients have undergone liver transplantation. One diabetic male developed NASH observed at biopsy performed for mild liver enzyme abnormalities 2 years after transplantation. This patient has subsequently progressed over 2 additional years to cirrhosis with loss of features of steatohepatitis at laparoscopic biopsy performed for the development of ascites.
Histology. Cirrhosis was definitively identified in the biopsy specimens from all but 2 patients who had advanced bridging fibrosis and clinical findings of cirrhosis, suggesting sampling error in the biopsy. None had features such as lymphoid aggregates or plasma cell infiltration to suggest a viral or autoimmune process. Two of the cases demonstrated occasional features consistent with focal steatohepatitis in the setting of otherwise-unremarkable regenerative nodules. Seventeen cases had simple steatosis in less than 30% of the hepatocytes, and 3 cases had more substantial steatosis without features of steatohepatitis. Although suspicious for associated NASH, these findings had been deemed as inadequate to make this diagnosis during the patient’s initial evaluation. Thirteen of the cases had no inflammatory activity, 24 cases demonstrated minimal activity, and 4 cases had mild activity. The inflammatory infiltrate in the latter consisted predominantly of small lymphocytes in fibrous bands. Mallory hyaline was present in 6, but only 2 of these had associated macrosteatosis (<30% in both), and none were felt to meet criteria diagnostic of NASH.
Comparison Groups. Because diabetes may be secondarily associated with cirrhosis,31 we determined the prevalence of diabetes mellitus in 39 age-selected nonalcoholic patients with cirrhosis from hepatitis C and 33 consecutive cirrhotic patients with PBC. Both diabetes mellitus and obesity were significantly more common in the cryptogenic and NASH patients compared with either the patients with cirrhosis caused by hepatitis C or those with cirrhosis caused by PBC (table 3). There was no significant difference in diabetes or obesity prevalence between the cryptogenic group and the NASH patients, although the NASH patients were significantly younger than the cryptogenic cirrhosis patients by approximately 10 years. These comparisons are summarized in table 3.
| table 3. Comparison of Cryptogenic Patients With Controls |
DISCUSSION
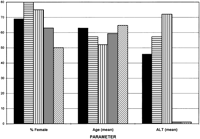
We observed that cryptogenic cirrhosis in this region is largely a disease of older females who present with mild or no liver enzyme abnormalities. This age and gender pattern is similar to prior studies of cryptogenic cirrhosis in other regions (Fig. 1).1,2,32,33 Although there is some diversity in the data presented in this report, we found that obesity and Type 2 diabetes were the most prevalent risk factors in cryptogenic cirrhosis. This suggests that many patients with cryptogenic cirrhosis represent advanced NASH.
| Fig. 1. Current ( |
A relationship between Type 2 diabetes, obesity, and cirrhosis has been debated in the past,34,35 although there has been less debate about an association between Type 2 diabetes, obesity, and NASH.9 The similarities between the patients in the present study and those often reported in prior reports of NASH suggest a common pathogenesis. As in cryptogenic cirrhosis, most patients with NASH are females with either Type 2 diabetes and/or obesity with minor liver enzyme abnormalities.3,5,10,12,36-39 The ratio of aspartate transaminase (AST):alanine transaminase (ALT) in NASH has been noted to change from <1.0 in uncomplicated NASH to >1.0 when cirrhosis is present.10We observed a similar pattern (table 3) in cryptogenic cirrhosis compared with patients with NASH (AST/ALT ratio = 1.5 ± 0.9 vs. 0.9 ± 0.4; P < .001). The mean age of our NASH patients was significantly younger (49 ± 14 vs. 63 ± 11 years) than our patients with cryptogenic cirrhosis (P < .005). This is similar to reported mean ages of NASH patients in other series (average, 51 years)3,5,10,12,36-39compared with that of cryptogenic cirrhosis patients (average, 58 years),1,2,32,33,40 consistent with a suspected progression of NASH to cirrhosis over a period of years.
The prevalence of Type 2 diabetes in the United States among individuals aged 45 to 74 years is lower than that observed in our patients: 12% versus 53%, respectively.41 It is possible that diabetes in our patients was secondary to the metabolic changes of cirrhosis, but this seems less likely because we observed significantly less diabetes in similarly aged patients with cirrhosis caused by either hepatitis C or PBC (table 3). It is difficult to compare the prevalence of obesity in our patients with the national average. BMI exceeding 30 is seen in about 30% of Caucasian patients aged 50 to 69, and up to 48% of African-American females aged 50 to 69, according to unpublished data from NHANES III.42 In comparison, we observed marked obesity (BMI > 31.1 for males and 32.3 for females) in 47% of our cryptogenic patients. That all of our cryptogenic cirrhosis and NASH patients were caucasian seems striking given our otherwise ethnically mixed patient population and the known increased prevalence of obesity in Americans of predominantly African descent.20
Nineteen patients had a history of distant blood transfusion. The absence of a frank bout of icteric hepatitis or known chronic hepatitis between the transfusion and the discovery of cirrhosis could represent subclinical disease or the absence a viral agent. We could not detect clinical or histological features that clearly distinguished patients with prior blood transfusions or indeterminant autoimmune scores from those with only risk factors for NASH. Indeed, the majority of previously transfused patients and those with indeterminant IAH scores had risk factors for steatohepatitis. Consistent with prior reports, we also could not detect a significant role for hepatitis G.43-45 The absence of findings suggestive of occult virus or autoimmune hepatitis is similar to another study of cryptogenic cirrhosis in patients undergoing liver transplantation.17
We observed ANA in all risk subgroups, although more commonly in the group with indeterminant autoimmune scores (table 1). However, positive antinuclear antibody alone is nonspecific and frequently seen in cryptogenic cirrhosis,17 as well as steatohepatitis.5,46 Its high prevalence in the indeterminant autoimmune score group could be artifactual as a result of the criteria of the score. Several HLA haplotypes have been associated with autoimmune hepatitis.30,47Compared with the studies summarized by Manns,30 we could not detect a prevailing HLA pattern suggestive of underlying autoimmune disease, although our sample size was small.
We observed a positive family history of unexplained liver disease in 13 patients (19%), including 2 with female relatives suffering from diabetes, obesity, and cirrhosis. Unexplained familial forms of cirrhosis have been previously described.48 However, under-reporting of cirrhosis in the family history is a potentially important and unexplored facet of this disease. We suspect that this may be a recurrent problem because of the common association between cirrhosis and alcohol abuse. We informally polled 20 of the patients in this study about misconceptions in their local community regarding the cause of their disease. Five (25%) patients reported encountering such misconceptions. In this setting, accurate family history is likely to be hampered by under-reporting to avoid a potentially embarrassing diagnosis.
Loss of substantial fatty infiltration has been previously observed in serial biopsies of NASH patients with progression to cirrhosis.3,11 Loss of hepatic fat and obvious steatohepatitis could result from sinusoidal capillarization in cirrhosis, which impairs the movement of large proteins,49 such as gut-derived lipoproteins into the liver and portosystemic shunting, which diverts blood-borne lipids away from the liver.50 In support of this hypothesis, changes in vascular flow within the liver have been shown to be responsible for focal sparing of segment 4 of otherwise-fatty livers.51 The development of histological NASH 2 years after transplantation in 1 of our diabetic, cryptogenic patients and subsequent progression after 2 more years to nonsteatotic “bland” cirrhosis is consistent with this type of progression.
In summary, we found that the majority of our cryptogenic cirrhosis patients are older females with Type 2 diabetes mellitus and current or past obesity. Clinical signs and symptoms in these patients are often subtle. While some patients may have unrecognized silent autoimmune hepatitis, an unidentified viral hepatitis, or occult alcohol-related liver injury, our data support progression of NASH as the more likely cause. These results suggest that fatty liver should be regarded as a potentially “guilty party” as opposed to “innocent bystander” in many patients with this condition.52 Further studies are necessary to confirm our observations and explore possible mechanisms.
Acknowledgment
The authors thank Andersen J. Yun, M.S., and Jungsuh P. Kim, Ph.D., of Genelabs Technology, Inc., Redwood City, CA, for performance of hepatitis G assays; James Patrie for assistance with the statistical analysis; and Katherine Begley and Kellie Williams for secretarial support.
Abbreviations:
NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; ANA, antinuclear antibodies; IAH, International Autoimmune Hepatitis score; BMI, body mass index; AST, aspartate transaminase; ALT, alanine transaminase.
FOOTNOTES
Received July 13, 1998; accepted November 25, 1998.
Address reprint requests to: Dr. Stephen H. Caldwell, Division of Gastroenterology and Hepatology, Box 145, University of Virginia Health Sciences Center, Charlottesville, VA 22908. E-mail:shc5c@virginia.edu; fax: (804) 924-0491.
REFERENCES
Copyright © 1999 by the American Association for the Study of Liver Diseases.