An Appraisal of Percutaneous Treatment of Liver Metastases
Vol. 4, Issue 4, pp. 271-275, July 1998
Alighieri Mazziotti,
From the Clinica Chirurgica 2, Policlinico S. Orsola, University of Bologna, Italy.
Abstract
Percutaneous treatments, such as ethanol injection and radio frequency, have been recently proposed for the treatment of liver metastases. The aim of this study was to evaluate the effects of these treatments in a series of 8 patients who subsequently underwent liver resection. These patients had been treated with percutaneous methods between December 1995 and May 1997. In 6 patients, the primary tumor was colonic; in 2 patients, carcinoid; and in 1 patient, ileal leiomyosarcoma. The lesions were all initially small in size (1.5 to 3.5 cm), single in 7 patients, and multiple in 1 patient with a carcinoid tumor. The initial decision for percutaneous treatment had been made on subjective grounds by the radiologists who originally saw the patients. The number of percutaneous treatment sessions ranged from 2 to 21. In all patients, a progression of the disease occurred. Four patients underwent a right hepatectomy; 1 patient, a left lobectomy; 2 patients, a segmentectomy; and 1 patient, a wedge resection. There was no operative mortality in any of these 8 patients. Two patients presented with seeding of the neoplasm on the diaphragm, which was resected. Histologic examination of all surgical specimens revealed the presence of vital neoplastic tissue; only two specimens of carcinoid tumors showed more than 50% necrosis of the nodules treated percutaneously.These results led us to express doubts as to the efficacy of percutaneous ablative treatment for liver metastases.
Introduction
The results of percutaneous treatment performed in hepatocellular carcinoma have led clinicians to extend the use of these treatments as therapy for liver metastases. Various clinical series have been published on the use of percutaneous ethanol injection (PEI) and, more recently, radiofrequency (RF) in the treatment of metastases as an alternative to surgery for single and small lesions, with results that are defined as “highly encouraging.”1-5
Patients initially treated with percutaneous methods are being referred to our center with increasing frequency. In this study, we present a series of 8 patients initially treated with PEI (5 patients) or RF (3 patients), or with both treatments (1 patient), of 255 patients who underwent surgery for liver resections for metastases between February 1996 and August 1997. The aim of this paper was to evaluate the effects and the problems of percutaneous treatment. We also report data relative to our overall experience of liver resections for metastases, which will offer a starting point for the discussion on the therapeutic strategies of liver metastases.
Materials and Methods
The 8 patients evaluated in this study, 3 women and 5 men, were between 56 and 64 years of age. In 5 patients, the metastases originated from a colonic tumor; in 2 patients, from an intestinal carcinoid; and in 1 patient, from an ileal leiomyosarcoma. In 7 patients, the lesions were initially single, whereas in 1 patient with carcinoid metastases, there were six intrahepatic lesions in both lobes. In 7 patients, the metastases were metachronous, and in 1 patient, the lesion was synchronous with respect to the primary tumor. In all patients, the preoperative workup excluded signs of extrahepatic, pulmonary, or lymph node diffusion of the tumor. In all patients, the primary tumor had been previously removed with radical surgery. The percutaneous treatments had been performed in other centers in all patients. In 7 patients, it had been selected as a therapeutic choice on subjective grounds by the radiologists who initially saw the patients and, in one case, at the request of the patient who initially refused to undergo surgery.
At the start of the percutaneous treatment, the hepatic lesions were small, with an average diameter of 2.5 cm (range, 1.5 to 3.5 cm). They were single in 7 patients and multiple in 1 patient with a carcinoid tumor. The lesions were localized in the right lobe in 6 patients, in the left lobe in 1 patient, and in both lobes in 1 patient. The number of percutaneous treatment sessions ranged from 2 to 21 cycles for PEI and from 2 to 3 cycles for RF. Resective surgery was performed at an average time of 6 months after the percutaneous treatment (range, 3 to 12 months). Only 1 patient had received systemic chemotherapy in this interval. One patient had received both PEI and RF. Before the hepatectomy, all patients underwent a chest and abdominal computerized tomographic (CT) scan, and 6 patients who underwent surgery for colonic resection underwent colonoscopy to exclude intestinal recurrence.
In all patients, surgery was proposed because the CT scan revealed a progression of the tumor, defined as an increase in the volume of the mass or the presence of areas with enhancement of the contrast medium at the edges of the lesions. Another 247 patients underwent liver resection in the period between 1982 and August1997 for metastases from colorectal tumors (184 patients) or tumors from other sites (163 patients).
Results
All patients treated with percutaneous therapy showed a progression of the disease on the CT scan. Four patients underwent right hepatectomies, 1 patient underwent a left lobectomy, 2 patients underwent segmentary resections, and 1 patient underwent a wedge resection. In 4 patients, a portion of diaphragm infiltrated bythe neoplasm was also resected. One patient with carcinoid liver metastases underwent a two-stage hepatectomy, consisting firstof a right hepatectomy with wedge resection on segment IV andresection of the diaphragm, and subsequently of a left lobectomy40 days later.
The typical procedure was performed for the right hepatectomy, together with vascular portal exclusion of the right hepatic vein before parenchymal division.6 In the patients who underwent a wedge resection or segmentectomy, vascular exclusion of onehemiliver6 was performed to minimize blood loss during resection. All the operations were performed without the need for blood transfusions.
Postoperative mortality in this series was zero. No patient presented major postoperative complications and the mean postoperative period of hospitalization was 8 days (range, 7 to 14 days). Inthe overall series of liver resections for metastases, postoperativemortality was 0.4% (1 patient died in 1984 as a result of hemorrhage from a peptic ulcer). Morbidity and long-term results are reported in detail in other publications.6,7
The cytonecrotic effect connected with percutaneous treatments was evaluated before surgery in all patients by CT scan and in 4 patients by magnetic resonance imaging. The surgical specimen was examined to evaluate the percentage of necrosis and the presence of vascular infiltration on the surrounding parenchyma. In all patients, the preoperative examination showed a progression of the neoplastic disease with an evident increase in volume of the lesion (6 patients) or with the appearance of hypodense areas with enhancement of the contrast medium at the edges of the lesion(2 patients). In 3 patients, the appearance of other surrounding neoplastic nodules was also observed. The histologic examination also showed the presence of vital neoplastic tissue in all specimens. The percentage of necrosis was correlated with the histologic type of the tumor and with the interval that had elapsed between the percutaneous treatment and liver resection. Only the patients with carcinoid metastases, treated respectively with PEI and with RF, presented necrosis of 80% in 1 patient and 50% in the other. In the 6 patients with colonic tumor metastases, partial (less than 20% of the neoplastic mass) areas of necrosis alongside vital neoplastic tissue were present in 2 patients, whereas in 4 patients, the lesions showed no necrotic areas. In these latter cases, surgery was performed more than 3 months after the conclusion of the percutaneous treatment. Three patients presented multiple satellite nodules adjacent to the tumor and signs of microvascular infiltration in the surrounding parenchyma.
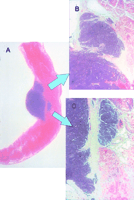
A portion of the diaphragm infiltrated by the neoplasm was resected in 4 patients at hepatectomy according to the technique already described.6 In 2 patients, the infiltration was because of close contact with the tumor, and in 2 patients, small neoplastic nodules were present in the lateral portions of the diaphragm in areas in which there was no infiltration of Glisson’s capsule(Fig.1). Both these patients had been treated with multiple sessions of RF, and the infiltration of the diaphragm was interpreted as a seeding of neoplastic cells along the pathway of the radio frequency probe.
| Figure 1. Diaphragmatic seeding from a carcinoid tumor in a patient who had undergone multiple sessions of PEI and RF. Various diaphragmatic nodules measuring 0.2 to 0.5 cm were detected at surgery on the lateral portions of the diaphragm in areas in which the neoplasm had not affected Glisson’s capsule. (A) H&E stain of macrosection of the nodule and a portion of the diaphragm. (B, C) Detail of the neoplastic infiltration between the muscular fibers. |
During follow-up, 2 patients died of tumor recurrence. The first patient, who underwent surgery in February 1996, developed a tumor recurrence in the remaining liver 6 months later whileundergoing chemotherapy and died after 14 months. This was a metastasis from cancer of the colon that, on initial presentation, measured 2 cm. After four sessions of ethanolinjection, the nodule had reached a diameter of 5 cm, with the appearance of other nodules in the adjacent segments. Surgery consisted of a right hepatectomy with resection of the diaphragm. The second patient, who underwent surgery in January 1995, developed a recurrence 7 months later and died after 13 months (table 1). All the remaining patients are alive, although 1 patient has an intrahepatic recurrence.
| To View This table | table 1. Data Summary of the Study Patients |
Discussion
In recent years, there has been a great increase in the use of percutaneous treatment of hepatocellular carcinoma on cirrhosis. PEI has been the most widely used method in a large number of clinical series from both the Far East and Europe.8,9 The limitations of resective surgery in cirrhotic patients justify the recourse to alternative treatments that are appropriate for the particular characteristics of hepatocellular carcinoma on cirrhosis, i.e., hypervascularized nodules, usually with a pseudocapsule and less consistency compared with the surrounding parenchyma, all favoring the spread of the ethanol inside the lesion and the cytonecrotic effect.
The results of percutaneous treatment obtained in hepatocellular carcinoma have led to an extension of the indications to include liver metastases.1-3 In addition to PEI, RF has more recently been introduced. This method determines a coagulative necrosis of the tissues by means of heat.4,5,10 In the larger series, which consisted of only several dozen cases, the results were defined as “potentially encouraging,” because complete necrosis (evaluated by radiology) is observed in about 50% of the lesions and a partial necrosis in the remaining lesions, both after PEI1,3and after RF.4,5 The response to the treatment was inversely proportional to the diameter of the lesions: A complete response was observed almost exclusively in lesions smaller than 3 cm, whereas all the larger lesions showed only partial necrosis. The long-term results of percutaneous treatments remain difficult to evaluate in view of the generally small number of patients treated and the short follow-up reported in the studies published so far. In the study that reported a longer follow-up,3 all the patients with colorectal metastases died within 46 months,regardless of the partial or total necrosis achieved by PEI. Onlymetastases from neuroendocrine tumors, which are well known to have a better prognosis,11 improve the overall results of this series. The two most recently published studies on RF4,5reported an even shorter follow-up, ranging from 6 to 18.1 months. These periods were totally insufficient to assess the long-term efficacy of treatment of liver metastases. It should be remembered that in untreated patients with small liver metastases (like those included in the studies mentioned here), the historic spontaneous survival rate is approximately 100% at 1 year12,13; we are therefore amazed that some patients with such initially small tumors died of neoplastic dissemination a little more than 1 year after treatment in the reported series.5
The most recent surgical series of liver resection for metastases showed that this operation is completely safe, with a mortality rate that has decreased to approximately 1%7,14-17 and was only 0.4% in a series of 255 liver resections in our overall experience.6
As to the long-term results of the surgical treatment of liver metastases, several factors are involved, such as the size and number of the lesions and the staging of the primary tumor.18 Apart from these prognostic factors, which depend on the tumor, another factor that is commonly agreed to be fundamental in surgery of liver metastases depends on the surgical approach itself and consists of an adequate margin of healthy tissue around the neoplasm. Only radical operations, with a margin of at least 1 cm, may offer hope of recovery and ensure long-term survival18; metastasectomies or cytoreductive surgery does not modify the patient’s prognosis in any way. A partial response or the idea of cytoreduction makes no sense when discussing the treatment of liver metastases.
This small series of liver resections in patients who had previously undergone percutaneous treatment led us to express doubt about the long-term efficacy of such treatment. The lesions treated were on initial presentation small and mostly single. At the time of surgery, the lesions had increased in size in all cases. In 2 patients, the diaphragm was infiltrated in an area in which there was no corresponding extension of the tumor on Glisson’s capsule, and the infiltration corresponded to a tumoral seeding along the pathway of the needle. In all these patients, the histologic examination of the resected specimen revealed the presence of vital tumoral tissue. Areas of necrotic tissue were observed in only two cases of carcinoid metastases, which show a different behavior with respect to adenocarcinoma metastases, the former being highly vascularized and sensitive to other locoregional treatments that induce ischemia.11
Thus, the hope that “what is not cured by the knife is cured by fire”19 seems to be unfulfilled in liver metastases. For single, small metastases, surgery must still be considered the gold standard and efforts must aim at constant follow-up of patients undergoing surgery for colorectal tumors so that early diagnosis and prompt treatment can be achieved.
Footnotes
Address reprint requests to Prof Alighieri Mazziotti, Clinica Chirurgica 2, Policlinico S. Orsola, Via Massarenti 9, 40138 Bologna, Italy.
References
| 1. | Livraghi T, Vettori C, Lattaroni S. Liver metastases: Results of percutaneous ethanol injection in 14 patients. Radiology 1991;179:709-712 [Medline] |
| 2. | Salim AS. Pilot study on alcohol-induced chemonecrosis of hepatic metastases from colonic cancer. HBP Surgery 1993;7:33-41 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 3. | Giovannini M, Seitz JF. Ultrasound-guided percutaneous alcohol injection of small liver metastases. Cancer 1994;73:294-297 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 4. | Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 1997;202:205-210 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 5. | Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: Treatment and follow-up in 16 patients. Radiology 1997;202:195-203 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 6. | Mazziotti A, Cavallari A, (eds). Techniques in liver surgery. London: Greenwich Medical Media, 1997. |
| 7. | Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, et al. Liver resection without blood transfusions. Br J Surg 1995;82:1105-1110 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 8. | Shen JC, Sung L, Huang GT, Chen GT, Yang PM, Lai MY. Intratumoral injection of absolute ethanol under ultrasound guidance for the treatment of small hepatocellular carcinoma. Hepatogastroenterology 1987;34:255-261 [Medline] |
| 9. | Livraghi T, Bolondi L, Buscarini L, Cottone M, Mazziotti A, Morabito A, et al. No treatment, resection and ethanol injection in hepatocellular carcinoma in a retrospective analysis of survival in 391 patients with cirrhosis. J Hepatol 1995;15:77-80 |
| 10. | Curley SA, Davidson BS, Fleming RY, Izzo F, Stephens LC, Tinckey P, et al. Laparoscopically guided bipolar radiofrequency ablation of areas of porcine liver. Surg Endosc 1997;11:729-733 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 11. | Wallace S, Ajani JA, Charnsagevej C, Du Brow R, Yang DJ, Chuang VP, et al. Carcinoid tumors: Imaging procedures and interventional radiology. World J Surg 1996;20:147-156 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 12. | Bengtsson G, Carlsson G, Hafstrom L, Jonsson PE. The natural history of patients with untreated liver metastases from colorectal cancer. Am J Surg 1981;141:586-589 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 13. | Wanebo HJ. Natural history of colorectal cancer metastasized to the liver. In: Wanebo HJ (ed). Hepatic and biliary cancer. New York: Marcel Dekker, 1987:464-466. |
| 14. | Belghiti J, Di Carlo I, Sauvanet A, Uribe M, Fekete F. A ten years experience with hepatic resection in 338 patients: Evolution in indication and operative mortality. Eur J Surg 1994;160:277-282 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 15. | Miyagawa S, Makunki M, Kawasaky J, Kakazu T. Criteria for safe hepatic resection. Am J Surg 1995;169:589-594 [ Link previously at www.ncbi.nlm.nih.gov ]. |
| 16. | Rees M, Plant G, Wells J, Bygrave S. One hundred and fifty hepatic resections: Evolution of technique towards bloodless surgery. Br J Surg 1996;83:1526-1529 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 17. | Nuzzo G, Giuliante F, Giovannini I, Tebela GD, Clement G, Vellone M, et al. Resection of hepatic metastases from colorectal cancer. Hepatogastroenterology 1997;15:751-759. |
| 18. | Scheele J, Stang R, Alterndorf HA, Hoffman A, Paul M, et al. Resections for colorectal liver metastases. World J Surg 1995;19:59-71 [ Link previously at www.ncbi.nlm.nih.gov ] |
| 19. | Hippocrates. Corpus. Kos. 400 B.C. |
Copyright © 1998 by the American Association for the Study of Liver Diseases