HEPATOLOGY, May 1998, p. 1421-1427, Vol. 27, No. 5
Neurovisual Impairment: A Frequent Complication of Alpha-Interferon Treatment in Chronic Viral Hepatitis
Emanuel K. Manesis1, Michael Moschos3, Dimitrios Brouzas2, John Kotsiras3, Constantine Petrou2, George Theodosiadis3, and Stephanos Hadziyannis1
From the 1 Academic Department of Medicine and the 2 Ophthalmology Clinic, Hippokration General Hospital,3 University Ophthalmology Clinic, Athens State General Hospital, Athens, Greece
ABSTRACT
Following our earlier observation of clinically evident optic tract neuropathy in patients receiving low-dose interferon (IFN) therapy, we prospectively evaluated 53 consecutive patients treated for chronic hepatitis B or C with a median dose of 3 MU of IFN-a2b thrice weekly. Measurements included routine ophthalmologic evaluation and recordings of visual evoked responses (VER), electro-retinograms (ERG), visual acuity, and visual fields, before, at the end of IFN treatment, and at follow-up visits. Baseline P100 latencies of VERs (base-VER) were abnormally prolonged in 24 patients (32 of 106 eyes, 30.2%); age was the only significant covariate associated with increased risk for an abnormal base-VER by multiple logistic regression (relative risk [RR] 5.3 per each 5-year increase in age). In 45 patients (74 eyes) with normal baseline P100 latencies, the end-of-treatment VERs (end-VER) were significantly prolonged compared with baseline, becoming abnormal in 11 (15 of 74 eyes, 20.3%) (138.8 ± 8.7 vs. 117.7 ± 5.2 ms, P < .001). This subgroup had older age (59.1 ± 11.0 vs. 47.5 ± 15.3, P = .007) and reduced visual sensitivity compared with their own pretreatment measurements (24.5 ± 1.6 vs. 23.0 ± 1.2db, P = .019). Changes of end-VERs by age had a sigmoid distribution with a steep increase of values beyond the 5th decade (R2 = .326, P < .001). In a logistic regression model, significant predictors of abnormal end-VERs were, patients’ age (RR 5.6 per each 5-year increase), presence of hepatitis B virus (HBV) infection (RR 15.1 compared with hepatitis C virus [HCV] infection) and serum cholesterol levels above 240 mg% (RR 7.1 compared with values <240 mg%). Subconjunctival hemorrhages were seen in 2 cases and funduscopic examination revealed cotton wool spots in one other. ERG recordings and the P100 amplitude remained unchanged. After stopping IFN, the treatment-associated neurovisual abnormalities reversed to normal in 7 patients (10 of 15 eyes) and persisted in 5 (5 of 15 eyes, 33.3%) for up to 37 (median 7.3) months observation, all patients remaining clinically asymptomatic. In conclusion, subclinical neurovisual impairment is a frequent, largely unrecognized complication of low-dose IFN therapy, and patients with chronic hepatitis B and older age appear to be most susceptible. This apparently innocuous complication is long lasting, possibly irreversible in some patients, with yet undetermined consequences on visual function. (HEPATOLOGY 1998;27:1421-1427.)
INTRODUCTION
Ocular side effects are infrequently reported during ![]() -interferon (IFN) therapy, including among else, cases of transient blurred vision,1 increased intraocular pressure,2 neovascular glaucoma,3 anterior ischemic optic neuropathy,4 retinal detachment,5 papilloedema,6 and eyeball rupture. 2,7 A better documented and apparently more frequent complication, especially in Japan, is IFN retinopathy, characterized by cotton wool spots, retinal hemorrhages, and microaneurysms occurring in an appreciable proportion of patients receiving high-dose IFN.8-12Following the recent description of symptomatic optic tract neuropathy in 3 of our patients treated with low-dose IFN for chronic viral hepatitis,13 we subjected all patients with viral hepatitis entering IFN treatment to ophthalmologic evaluation, including visual neurophysiologic measurements before, at the end of treatment, and at follow-up visits. We herein report our findings.
-interferon (IFN) therapy, including among else, cases of transient blurred vision,1 increased intraocular pressure,2 neovascular glaucoma,3 anterior ischemic optic neuropathy,4 retinal detachment,5 papilloedema,6 and eyeball rupture. 2,7 A better documented and apparently more frequent complication, especially in Japan, is IFN retinopathy, characterized by cotton wool spots, retinal hemorrhages, and microaneurysms occurring in an appreciable proportion of patients receiving high-dose IFN.8-12Following the recent description of symptomatic optic tract neuropathy in 3 of our patients treated with low-dose IFN for chronic viral hepatitis,13 we subjected all patients with viral hepatitis entering IFN treatment to ophthalmologic evaluation, including visual neurophysiologic measurements before, at the end of treatment, and at follow-up visits. We herein report our findings.
PATIENTS AND METHODS
Study Protocol. Patients entering IFN treatment for chronic hepatitis B or C between May 1994 and June 1996 were evaluated as candidates for the study. All had been followed at the Hepatology Outpatient Clinic of the Academic Department of Medicine in Athens, with biochemically and virologically active liver disease for at least 6 months and had had a liver biopsy, unless medically contraindicated. The diagnosis of hepatitis B required a positive hepatitis B surface antigen and anti-hepatitis B core radioimmunoassay tests (AUK-3, AMBI-COREK, Sorin Diagnostics, Torino, Italy), and hepatitis C, an anti-HCV positive test by a second generation enzyme-linked immunosorbent assay (Chiron Co., Emeryville, CA) confirmed by a second generation recombinant immunoblot assay (RIBA-2, Chiron Co., Emeryville, CA). Patients with autoimmune abnormalities or coinfected with hepatitis viruses B, C, or D or with the human immunodefficiency virus (HIV) were excluded. All candidates underwent ophthalmological screening and cases with eye disease precluding a reliable neurovisual assessment, for example, dense cataracts, impaired best-corrected near vision, visual field abnormalities, or glaucoma, were excluded. Patients passing the screening test were included in the study and subjected to measurements of the ophthalmic pressure and the visual acuity, fundus examination through a dilated pupil, assessment of visual fields, electroretinograms (ERG), and visual evoked responses (VER). The same ophthalmological measurements were repeated in all patients at the last month of IFN treatment. Clinical follow up included periodic visits during and after IFN treatment, in which any visual complaints were recorded. Symptomatic cases were referred and evaluated by an experienced ophthalmologist; those with abnormally prolonged neurovisual measurements were re-examined periodically following discontinuation of IFN.
Patients. Seventy-five consequent patients were screened to enter the study. Five of them were excluded: two cases for a previous cataract operation; one case with current presence of dense cataracts impairing best-corrected near vision; one for an extensively myopic fundus; and one case with a past history of partial central artery thrombosis and current bilateral paracentral relative scotomas. The remaining 70 patients had a baseline ophthalmological and neurovisual evaluation and they started IFN treatment. Seventeen of them did not appear for a second neurovisual assessment after completing their IFN course and they were, therefore, excluded from the study. Fifty-three patients completed the protocol and were included in the study. The demographic, clinical, and laboratory characteristics of the studied group are shown in table 1. The mean age was 52.5 ± 14.4 years (median 55, range 16-74); 50.9% were males. Overall, chronic hepatitis C predominated slightly (58.5%) and 24 of the cases (45.3%) had cirrhosis (Child-Pugh A in 23 of 24). Thirteen patients (24.5%) had mild hypertension; 4 (7.5%) type-II diabetes; and 4 additional patients (7.5%) had a normal oral glucose tolerance test. Three patients (5.7%) had hypercholesterolemia (serum cholesterol ![]() 240 mg%). In 9 patients (17%) the total cholesterol to high-density lipoprotein (HDL) ratio was above 5. All hepatitis B patients were hepatitis B e antigen negative and anti-hepatitis B e-positive.
240 mg%). In 9 patients (17%) the total cholesterol to high-density lipoprotein (HDL) ratio was above 5. All hepatitis B patients were hepatitis B e antigen negative and anti-hepatitis B e-positive.
| View This table | table 1. Clinical and Laboratory Characteristics of Patients Studied |
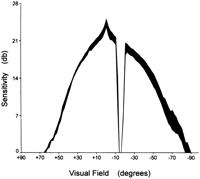
Methods. The neurophysiologic assessment of all subjects was performed by examiners who were unaware of the patients’ clinical status and according to criteria set by the American Electroencephalographic Society.14 The ERG and the VER were measured by a computerized EREV-99 apparatus (Lace Electronics Co., Italy). A pattern stimulation of 6-mm check size, equaling 55 min of subtending angle was used for VER measurements.15 The contrast of the pattern was 100%, the reversal frequency 2.08/s, and the band was filtered passing between 1 to 30 Hz. Fifty responses were averaged for each trace. The P100 implicit time (in ms) and the amplitude (in µV) of the VER were measured and further considered. The ERGs were elicited by flash stimulation through a white filter at 0.5 mJoule, 1 Hz frequency, and zero background intensity. Under these conditions, normal individuals, aged 58.1 ± 6.8 years (range, 38-70), elicited P100 implicit time VER responses of 115 ± 5.3 ms (range, 105-128.4) with an intersession coefficient of variation (CV) of 1.6%, mean P100 amplitude 7.20 ± 1.05 µV (range, 4.71-10.98), and mean ERG responses 54.4 ± 8.2 µV (range, 40.0-62.0). Abnormal values were considered those outside the ±2 SD range (VER > 127 ms, amplitude < 5.10 µV, and ERG < 38.0 µV). Visual fields were recorded using a Goldmann perimeter with a maximum stimulus intensity of 1000asb and a 31.6asb background. The standard sequence of stimulus strength was started from the 1 to 4 e and downwards following all the sequence until the last one the patient could see. Kinetic perimetry was performed and all isopters were drawn. Profile plots along the horizontal meridian were drawn and converted to decibel (db) sensitivity.16
Statistical Analysis. Patients were analyzed as individuals and as groups of eyes because the abnormal findings in several cases were unilateral. For univariate group comparisons the ![]() 2 or the Student’s t test were used, and for paired comparisons the paired t test. In all cases, tests of significance were two-tailed. Results are presented as mean ± SD or median (range), whenever appropriate. For multivariate analysis we developed a logistic regression model using a backward likelihood-ratio method, a simple treatment of all categorical variables and P = .05 for entry and P = .1 for removal of the independent variables (SPSS for Windows 95, version 7.5, SPSS Inc., Chicago, IL). Curve fitting was performed by the same computer program, selecting the regression line with the highest R2 among a number of mathematical transformations, including linear, logarithmic, logistic, quadratic, cubic, power, and exponential ones. Eyes with pretreatment abnormalities in VERs were analyzed separately. Central threshold visual sensitivity at 0° degrees was used for paired and group comparisons of the eyes.
2 or the Student’s t test were used, and for paired comparisons the paired t test. In all cases, tests of significance were two-tailed. Results are presented as mean ± SD or median (range), whenever appropriate. For multivariate analysis we developed a logistic regression model using a backward likelihood-ratio method, a simple treatment of all categorical variables and P = .05 for entry and P = .1 for removal of the independent variables (SPSS for Windows 95, version 7.5, SPSS Inc., Chicago, IL). Curve fitting was performed by the same computer program, selecting the regression line with the highest R2 among a number of mathematical transformations, including linear, logarithmic, logistic, quadratic, cubic, power, and exponential ones. Eyes with pretreatment abnormalities in VERs were analyzed separately. Central threshold visual sensitivity at 0° degrees was used for paired and group comparisons of the eyes.
The trial was approved by the Hospital’s Ethical Committee. Informed consent was obtained from all patients.
RESULTS
Baseline Neurovisual Data
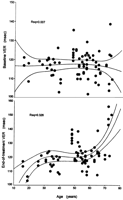
Before treatment mean VER values in all 53 patients (106 eyes) were 122.8 ± 10.9 ms (table 2). Prolongation of the base-VER above the upper limit of normal was observed in 24 of 53 patients (32 eyes, 30.2%; mean 136.0 ± 8.1 ms; range, 127.8-159.6) (table 3), being bilateral in 8 cases and unilateral in 16. Most of the patients with abnormally prolonged baseline VERs were older than 55 years (16 of 24 patients, 66.7%) and the mean age differed significantly to those with normal pretreatment VER values (57.3 ± 12.6 vs. 48.5 ± 15.0,P = .027). Their central visual sensitivity was also significantly reduced, compared with patients with normal base-VERs (24.2 ± 1.5 vs. 25.1 ± 1.0 db,P = .012). In multiple logistic regression analysis, among 12 independent pretreatment variables including, sex, age, viral etiology of liver disease, presence of cirrhosis, diabetes, hypertension, hypercholesterolemia (above or below the 240 mg% level), LDL cholesterol, the ratio of total serum cholesterol to high-density lipoprotein, smoking (in packs*years), serum aspartate aminotransferase, and platelet count, the only significant predictor of an abnormally elevated pretreatment VER (values above or below the 127-ms cutoff level) was age (P = .005, relative risk [RR] 5.3 per 5 years increase in age, 95% confidence intervals [CI] 5.1-5.5).
| View This table | table 2. Neurovisual Parameters in 53 Patients (106 Eyes) Studied Before and at the End of an Interferon Course |
| View This table | table 3. Effect of Interferon Treatment on Neurovisual Parameters of Eyes With Baseline Normal or Abnormal P100 Latencies of Visual Evoked Responses |
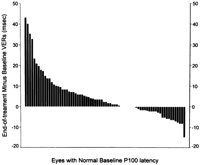
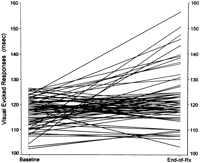
End-of-Treatment Data. A second neurovisual evaluation was carried out within 10.2 ± 3.4 months (median 11; range, 1.8-18.3 months) from the first evaluation, whereas the patients were still receiving IFN. Mean values of P100 latency of VER (end-VER) in the entire group of 53 patients (106 eyes) had significantly increased (126.3 ± 12.9 ms) compared with baseline values (122.8 ± 10.9 ms, P = .036) (table 2, Fig. 1). Because, as mentioned earlier, some of the patients receiving IFN have already had abnormal baseline VERs, we evaluated the on-treatment neurovisual findings separately for the 74 eyes (45 patients) with normal base-VER values and for the 32 eyes (24 patients) with abnormally prolonged baseline VERs (table 3).
|
Fig. 1. P100 latencies of visual evoked responses obtained before and at the end of interferon treatment in the group of 53 patients (106 eyes). Values were significantly prolonged during therapy. |
| View This table | table 4. P100 Latency and Central Visual Sensitivity at Baseline and at the End of Interferon Treatment of Eyes With Normal Pretreatment Visual and Neurophysiological Parameters |
Eyes With Abnormal Pretreatment Visual Evoked Responses. Group or paired-eye comparisons of the 32 eyes (24 patients) with abnormally prolonged baseline P100 latencies, did not reveal any significant changes among the base- and end-VER responses (136.0 ± 8.1 vs. 136.2 ± 12.8, P = NS). During therapy, in 10 eyes (10 patients) the P100 latency became longer by more than 8.1 ms (2 SD of the inter-session CV) compared with baseline values (137.2 ± 7.1 vs. 147.3 ± 13.1, P = .045), in 13 it remained unchanged and in 9 it became shorter by more than 8.1 ms (139.1 ± 11.9 vs. 128.7 ± 13.2, P = NS), falling within the normal range in 4 eyes (4 patients). Paired-eye comparisons of visual acuity and sensitivity before and during treatment in the 24 patients (32 eyes), demonstrated significant deterioration of respective measurements (table 3). Again, no significant changes were noted in the ERGs and the amplitudes before and during treatment.
Follow-Up of Patients. With current follow up of 9.8 ± 8.5 months (median 7.3; range, 1.8-37) after stopping the IFN, the observed on-treatment neurovisual abnormalities regressed to normal in 10 of 15 eyes (7 patients) and persisted in 5 (33.3%, 5 patients). The median time of the VER recovery was 4.8 months and the longest 37 months. The patient with the longest VER recovery became normal in one eye, whereas the other one still maintains an abnormal P100 latency. His current visual acuity is 9 of 10 at both eyes. All patients have remained asymptomatic.
Retinal Changes. At baseline, 15 patients had mild hypertensive and/or arteriosclerotic fundus changes, consisting of mild arteriolar wall thickening associated with or without venous compression and vascular tortuosity.18 Ten of the patients had a history of hypertension and 4 were diabetics. None of the latter had diabetic retinopathy.19 During IFN treatment, only one case developed cotton wool spots. The patient was a 45-year-old, non-hypertensive, non-diabetic male with chronic hepatitis B and abnormally prolonged pretreatment VERs and normal visual acuity. He developed cotton wool spots at the third month of treatment. Five months later, whereas still on IFN, the cotton wool spots were still there, the P100 latency was abnormal bilaterally but unchanged compared with baseline values and the visual acuity was 9 of 10 bilaterally. Subconjunctival hemorrhages were observed in 2 patients, both with chronic hepatitis C. The first was a 70-year-old female diabetic, hypertensive patient and the second a 38-year-old female with cirrhosis of Child-Pugh class C. Unilateral subconjunctival hemorrhages developed at the fourth and tenth month of treatment, respectively. In both cases hemorrhages disappeared, whereas treatment was being continued. In none of the patients with or without IFN-related VER abnormalities, microaneurysms, papilledema, scotomas, or increases in intraocular pressure were observed to develop under treatment.
DISCUSSION
The results of this study indicate that during IFN treatment, approximately 1 of 4 patients, normal otherwise at baseline, is expected to develop visual neurophysiologic abnormalities in the form of prolonged P100 latency of visual evoked potentials and a reduction in sensitivity in central vision. These abnormalities appear to be neither short lived, nor temporary, at least in some patients. Their presence raises several questions: what part of the optic apparatus or neural pathway is exactly affected? Are they caused by functional or by structural changes? What is their pathophysiology? Do they have any clinical significance? Our data do not permit exact answers but allow some inferences to be made.
The long duration of the recovery phase, for patients who did recover, and the existence of a considerable fraction of patients who never did, indicate that the underlying visual abnormality has a structural rather than a functional background. The retina is an already established site of interferon toxicity in both humans8-10 and experimental animals,19 with development of cotton wool spots, hemorrhages, and microaneurysms. However, in this study, among the 74 eyes with baseline normal neurovisual parameters no cases of retinopathy were observed. This lack of funduscopic findings and the normal electroretinograms among the subgroup of patients who developed abnormal P100 latencies during IFN suggest that, retina as an anatomic or functional unit was not affected by IFN and the site of toxicity was probably located beyond that point.
Prolongation of visual evoked responses may express reduction of conductive velocity of the optic fibers. Such changes can appear before any clinical visual signs 20,21 and be suggestive of optic tract neuropathy.22 In fact, associated with IFN therapy, there have been case reports of optic neuropathy with visual loss and scotomas,13 as well as, reports of anterior ischemic optic neuropathy, characterized also by sudden visual loss, segmental optic disc edema, and disc-related field defects.4 However, none of our cases with prolonged VERs demonstrated scotomas, papilledema, or a subjective sense of diminished vision, although paired measurements of eyes before- and on-treatment did show reduction of visual sensitivity. The visual abnormalities reported in this paper, do not qualify for optic neuropathy or anterior ischemic optic neuropathy, but they may possibly represent earlier changes of the same pathophysiologic spectrum in which extreme and rare events are optic neuritis and anterior ischemic optic neuropathy.
The nature of pathophysiologic changes underlying the visual complications associated with IFN is not clear. The reported retinal lesions, including the presence of cotton wool spots, capillary nonperfusion, arteriolar occlusion, and retinal hemorrhages8-12 do support an ischemic mechanism. IFN is a multipotent biologic response modifier, suppressing and inducing different T-cell subsets and augmenting antibody responses and autoimmunity.23 It could conceivably induce ischemia in retinal or small vessels of the optic nerve through deposition of immune complexes and local inflammation. 24,25 IFN is also an antiangiogenic agent which is able to inhibit experimental intraocular neovascularization26 and clinically effective for Kaposi sarcoma27 and hemangiomas of the infancy.28 Such pharmacological action could conceivably contribute to ischemia in susceptible vascular beds.
Host factors could also be important in the pathogenesis of IFN-associated visual complications. Our study population was comprised of middle-aged patients, half of them, older than 55 years, and multivariate analysis isolated older age and hypercholesterolemia as significant predictors of neurovisual abnormalities developing during IFN treatment. Although conditions known to be associated with accelerated atherosclerotic processes and IFN retinopathy, such as diabetes and hypertension, 11,12 were not significant predictors in our multivariate model, it is still possible that vascular changes associated with older age made patients more susceptible to the visual adverse effects of IFN.
The underlying viral liver disease also merits consideration in relation to neurovisual findings reported in this paper. Chronic hepatitis B, and particularly hepatitis C, have been associated with a host of immunological abnormalities, including among else, arteritis, cryoglobulinemia, autoimmune thyroiditis, and thrombocytopenia. 29,30 Although ocular complications can possibly occur in this setting, we are aware of only one report of retrobulbar optic neuritis occuring in a patient with acute type B hepatitis not receiving IFN.31 In this case, onset of ocular symptoms was associated with activation of the classic and alternative complement pathways and high levels of circulating immune complexes. On the other hand, ocular evaluation in a cumulative group of 156 patients (most of them with chronic hepatitis C) from 3 recent prospective studies, failed to reveal any funduscopic or visual abnormalities before IFN treatment.10-12 In agreement with these findings, no fundus abnormalities were detected in any of our 53 patients at baseline, despite the presence of abnormally prolonged P100 latencies in 24 of them (32 of 106 eyes, 30.2%) and the significant suppression of their visual sensitivity. Cirrhosis of viral etiology, for ill-defined reasons, has been associated with prolonged visual evoked responses in 15% to 63% of the cases, even in the absence of encephalopathy. 21,32,33 In this study, cirrhosis was evenly distributed among patients with or without baseline VER changes and it was not a significant predictor of baseline VER abnormalities in the multivariate analysis model. Again, older age was found to be the only predictor of pretreatment neurovisual abnormalities and this finding needs further confirmation in larger cohort studies, including patients with chronic viral hepatitis and cirrhosis.
The type of viral hepatitis and particularly, HBV infection was found in the multivariate analysis to be 15 times more likely to be associated with neurovisual abnormalities during IFN treatment, compared with HCV infection. The mean age of HBV-positive patients was not significantly different compared to HCV-positive ones (50 vs. 54 years) and patients with HBV cirrhosis were significantly fewer compared with respective HCV cases (5 of 22 vs. 19 of 31, P = .0055). The reason of increased susceptibility for neurovascular abnormalities in patients with chronic hepatitis B who receive IFN is not apparent and requires further study.
In conclusion, we have reported that a significant proportion of patients, normal otherwise at baseline, develop neurovisual abnormalities in the form of prolonged VERs and reduced central visual sensitivity during interferon treatment. Older patients and those with HBV infection appear to be the most susceptible. Such visual changes may be present even without any morphologic evidence of retinopathy or anterior ischemic optic neuropathy and although subclinical, are long-lasting and possibly permanent in some cases. The clinical significance of these findings is undetermined at present, but they merit consideration as a potentially dangerous interferon-associated visual complication.
Footnotes
Abbreviations:
IFN, interferon; VER, visual evoked response; ERG, electroretinogram(s); RR, relative risk; HBV, hepatitis B virus; HCV, hepatitis C virus; CI, confidence interferon.
Received December 28, 1996; accepted February 3, 1998.
Address reprint requests to: Stephanos Hadziyannis, M.D., Academic Department of Medicine, Hippokration General Hospital, 114 Vas. Sophias Ave., Athens 115 27, Greece. Fax: 01-7706871.
REFERENCES
- Ene L, Gehenot M, Detry-Morel M, Geubel AP. Transient blurred vision after interferon for chronic hepatitis C [Letter]. Lancet 1994; 344: 827-828.
- Kuga K, Hasumura S, Nagamori S, Toda G, Kitahara K. Intraocular hemorrhage developing during interferon therapy. Intern Med 1996; 35: 15-18.
- Ayaki M. Development of neovascular glaucoma in the course of interferon alfa therapy for hepatitis type C [Letter]. Br J Ophthalmol 1994; 78: 238
- Purvin VA. Anterior ischemic optic neuropathy secondary to interferon alfa. Arch Ophthalmol 1995; 113: 1041-1044
- Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol 1996; 25: 283-291
- Farkkila M, Iivanainen M, Roine R, Bergstrom L, Laaksonen R, Niemi ML, Cantell K. Neurotoxic and other side effects of high-dose interferon in amyotrophic lateral sclerosis. Acta Neurol Scand 1984; 70: 42-46
- Yamanda H, Mizobuchi K, Isogai Y. Acute onset of ocular complications with interferon [Letter]. Lancet 1994; 343: 914
- Guyer DR, Tiedeman J, Yannuzzi LA, Slakter JS, Parke D, Kelly J, Tang RA, et al. Interferon-associated retinopathy. Arch Opthalmol 1993; 111: 350-356
- Sugano S, Yanagimoto M, Suzuki T, Sato M, Onmura H, Aizawa H, Makino, et al. Retinal complications with elevated circulating plasma C5a associated with interferon-alpha therapy for chronic active hepatitis C. Am J Gastroenterol 1994; 89: 2054-2056
- Hayasaka S, Fujii M, Yamamoto Y, Noda S, Kurome H, Sasaki M. Retinopathy and subconjunctival haemorrhage in patients with chronic viral hepatitis receiving interferon alfa. Br J Ophthalmol 1995; 79: 150-152
- Soushi S, Kobajashi SS, Obazawa H, Kigasawa K, Shiraishi K, Itakura M, Matsuzaki S. Evaluation of risk factors of interferon-associated retinopathy in patients with type C chronic active hepatitis. Nippon Ganka Gakkai Zasshi 1996; 100: 69-76
- Kawano T, Shigehira M, Uto H, Nakama T, Kato J, Hayashi K, Maruyama T, et al. Retinal complications during interferon therapy for chronic hepatitis C. Am J Gastroenterol 1996; 91: 309-313
- Manesis EK, Petrou K, Brouzas D, Hadziyannis S. Optic tract neuropathy complicating low-dose interferon treatment. J Hepatol 1994; 21: 474-477
- American Electroencephalographic Society. Guidelines for clinical evoked potential studies. J Clin Neurophysiol 1984;1:3-53.
- Chiapa KH, Gill EM, Lentz KE. The effect of check size on P100 latency. Electroencephalogr Clin Neurophysiol 1985; 61: 29P-30P
- Aderson DR. Perimetry with and without automation. Mosby Co, St Louis, Ed. 2. 1987.
- Kanski JJ. Hypertensive retinopathy. In: Clinical Ophthalmology. Ed. 2. A Systematic Approach. London: Butterworths, 1989:328-330.
- Klein R, Klein BEK. Diabetic eye disease. Lancet 1997; 350: 197-204
- Kertes PJ, Britton Jr WA, Addison DJ, Munro SM, Marshall DH, Leonard BC. Toxicity of intravitreal interferon alpha-2b in the rabbit. Can J Ophthalmol 1995; 30: 355-359
- Woung LC, Jou JR, Liaw SL. Visual function in recovered ethambutol optic neuropathy. J Ocul Pharmacol Ther 1995; 11: 411-419
- Weissenborn K, Scholz M, Hinrichs H, Wiltfang J, Schmidt FW, Kunkel H. Neurophysiological assessment of early hepatic encephalopathy. Electroencephalogr Clin Neurophysiol 1990; 75: 289-295
- Halliday AM, McDonald WI, Mushin J. Delayed visual evoked response in optic neuritis. Lancet 1972; 1: 982-985
- Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. 1997;112:1017-1021.
- Fleet WS, Watson RT. Autoimmune optic neuritis: a potentially treatable form of visual loss. Ann Ophthalmol 1986; 18: 144-146
- Kupersmith MJ, Burde RM, Warren FA, Klingele TG, Frohman LP, Mitnick H. Autoimmune optic neuropathy: evaluation and treatment. J Neurol Neurosurg Psychiatr 1988; 51: 1381-1386
- Miller J, Stinson WG, Folkman J. Regression of experimental iris neovascularization with systemic alpha-interferon. Ophthalmology 1993; 100: 0-14
- Abrams DI, Volberding PA. Alpha interferon therapy of AIDS-associated Kaposi’s sarcoma. Semin Oncol 1986; 13: 43-47
- White CW, Sonbeimer HM, Crouch EC, Wilson H, Fan LL. Treatment of pulmonary hemangiomatosis with recombinant interferon alfa-2a. N Engl J Med 1989; 320: 1197-1200
- Hadziyannis SJ. The spectrum of extrahepatic manifestations of hepatitis C virus infection. J Vir Hep 1997; 4: 9-28
- Lisker-Melman M, Webb D, Di Bisceglie AM, Kassianidis C, Martin P, Rustgi V, Waggoner JG, et al. Glomerulonephritis caused by chronic hepatitis B virus infection: treatment with recombinant human alpha-interferon. Ann Intern Med 1989; 111: 479-483
- Galli M, Morelli R, Casellato A, Perna MC. Retrobulbar optic neuritis in a patient with acute type B hepatitis. J Neurol Sci 1986; 72: 195-200
- Levy LJ, Bolton RP, Losowsky MS. The use of the visual evoked potential (VEP) in delineating a state of subclinical hepatic encephalopathy. A comparison with the number connection test. J Hepatol 1987; 5: 211-217
- Quero JC, Schalm SW. Subclinical hepatic encephalopathy. Semin Liver Dis 1996; 16: 321-328
0270-9139/98/2705-0033$3.00/0
Copyright © 1998 by the American Association for the Study of Liver Diseases.