Hepatology, November 1998, p. 1416-1423, Vol. 28, No. 5
Histopathology of the Liver in Children With Chronic Hepatitis C Viral Infection
Kamran Badizadegan1, Maureen M. Jonas2, Mary Jane Ott2, Suzanne P. Nelson2, and Antonio R. Perez-Atayde1
From the 1Department of Pathology and 2Center for Childhood Liver Disease, Combined Program in Gastroenterology, Children’s Hospital and Harvard Medical School, Boston, MA.
ABSTRACT
Although the epidemiology, natural history, and pathological aspects of chronic Hepatitis C are well-defined in the adult population, little is known about the characteristics of chronic Hepatitis C infection in children. Reports on the histological features and progression of Hepatitis C in children are scarce, and consist primarily of multicenter studies in Japanese and European children. Given the geographic variations in viral genotype and the association of pathology with genotype, whether the Japanese and European studies can be extended to the North American populations is unclear. We report the histopathology of the liver in 40 children with chronic Hepatitis C infection treated in a single North American institution. The children included 19 males and 21 females ranging in age from 2.0 to 18.6 years at the time of liver biopsy (mean ± SD: 11.4 ± 4.3 years). Our findings indicate that the characteristic histopathological lesions of chronic Hepatitis C infection, including sinusoidal lymphocytosis, steatosis, portal lymphoid aggregates/follicles, and bile duct epithelial damage, occur with approximately the same frequencies in children as have been reported in adults. Necroinflammatory activity was generally mild. Portal fibrosis was present in 78% of the specimens, including fibrous portal expansion (26%), bridging fibrosis (22%), bridging fibrosis with architectural distortion (22%), and cirrhosis (8%). Centrilobular pericellular fibrosis, which has not been previously reported in the context of chronic Hepatitis C infection in adults or children, was also a prominent feature in our series, occurring with a similar frequency as steatosis or portal lymphoid aggregates/follicles. Our data suggest that in spite of mild histological necroinflammatory activity in general, the stage of fibrosis in children can be severe in spite of relatively short duration of infection. (HEPATOLOGY 1998;28:1416-1423.)
INTRODUCTION
Hepatitis C virus (HCV) infects nearly 4 million people in the United States, and is now the leading cause of liver transplantation in adults. Although it is likely that some of these adults acquired the infection during childhood, the number of children infected with HCV is unknown. In spite of extensive epidemiological data, natural history, and detailed characterization of the morphological features of chronic Hepatitis C infection in adults,1-9 little is known about the epidemiology, histology, and progression of chronic HCV infection in children. Available studies consist primarily of clinically oriented reports from Japan and Europe.10-17 Given the geographic variations in HCV genotypes,18,19 and the potential effect of genotype on pathology,20-26 whether the Japanese and European findings can be extrapolated to the infected American children is unclear.
The only series of pediatric Hepatitis C patients reported with special emphasis on the liver pathology is that of a multicenter study of Japanese children.27 Surprisingly, the stage of fibrosis in this cohort was mild, with only a 3.6% prevalence of bridging fibrosis with architectural distortion, and no cases of cirrhosis. This seemingly mild course of chronic Hepatitis C infection in children is in contrast with the findings in some of the earlier clinical reports in which the prevalence of cirrhosis in chronic Hepatitis C was found to be up to 14%.12,14,16 Here we report the histopathology of the liver in 40 children with chronic HCV infection who have been followed at our institution.
PATIENTS AND METHODS
Patients. All liver biopsies and hepatectomy specimens from children treated at the Boston Children’s Hospital for chronic HCV infection were reviewed. Patients were considered eligible for this study if they were: 1) 18 years of age or younger at the time of diagnosis; 2) had at least two alanine transaminase (ALT) determinations greater than 1.5 times upper limit of the reference range over at least a 6-month period; and 3) were seropositive for antibodies to HCV by the second-generation recombinant immunoblot assay and/or had HCV RNA by reverse-transcriptase polymerase chain reaction. All patients were seronegative for Hepatitis B surface antigen, and patients with concurrent other primary liver diseases were excluded from the study. The eligible children included 19 males and 21 females ranging in age from 2.0 to 18.6 years at the time of liver biopsy (mean ± SD: 11.4 ± 4.3 years). Other clinical characteristics of these children are summarized in table 1.
| table 1. Clinical Characteristics of Children With Chronic Hepatitis C Infection |
Materials. A total of 50 specimens including 48 core needle biopsies, 1 wedge biopsy, and 1 hepatectomy specimen were available for review. Four of the 48 needle biopsies were repeat biopsies from patients who had received interferon therapy for Hepatitis C. All specimens were fixed in formalin and embedded in paraffin. Four-micrometer-thick sections stained with hematoxylin-eosin and Masson trichrome were available for examination in all cases. In the majority of cases (85%), an additional panel of stains including Gomori reticulin, periodic acid-Schiff with and without diastase, and Prussian blue were available for examination. Immunohistochemical stain for cytokeratins (monoclonal antibody clone AE1, Signet Laboratories, Dedham, MA) specific for bile duct epithelium was performed in one case with centrilobular inflammation to confirm that the inflamed areas in question represented central veins rather than portal tracts. All specimens were examined by two pathologists, and classified by consensus for all abnormal histological findings. The histological activity index (or histological grade) of the disease in each case was determined using three different widely used schemes for evaluation of the inflammatory activity. The histological activity index for the METAVIR grading scheme was determined based on the published guidelines.28 The histological activity index for the Knodell and Scheuer schemes was expressed as the sum of scores for portal inflammation, lobular activity, and piecemeal necrosis, as appropriate.29,30 The extent of fibrosis (or histological stage) of the disease in each case was determined using the Knodell and Scheuer classification schemes.29,30
Statistical Analysis. All data analyses mentioned in the results section were performed using the StatView 4.5 statistical analysis package (Abacus Concepts, Inc., Berkeley, CA).
RESULTS
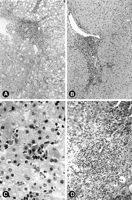
General Morphology. The characteristic histopathological findings of HCV infection, including bile duct epithelial damage, portal lymphoid aggregates and/or follicles, lymphocyte infiltration of the lobules, and steatosis,1-9 were present to some extent in each case (table 2, Fig. 1). Lymphocyte infiltration of the lobules was the most common feature, present in 74% of the biopsies, followed by steatosis (50%), portal lymphoid aggregates or follicles (48%), and bile duct damage (28%). Four needle biopsies obtained after interferon treatment showed minimal to no lobular inflammation or necroinflammatory activity. In general, histological changes indicating hepatocellular damage such as ballooning degeneration, reactive nuclear changes, and pseudoacinar transformation were minimal or absent. The only exception was one biopsy from a child who met the patient selection criteria, in whom the main histological finding was that of centrilobular hepatocellular damage with mild hepatocellular ballooning degeneration and occasional multinucleated hepatocytes.
| View This table | table 2. Summary of Histopathological Findings |
| Fig. 1. Histological characteristics of chronic HCV. (A) Portal inflammation with dense lymphoid aggregate, interphase necroinflammatory activity, and moderate macro-and microvesicular steatosis. (B) Periportal necroinflammatory activity (piecemeal necrosis) was usually mild. (C) Focus of lobular lymphocyte-mediated necroinflammatory activity with a cluster of dying hepatocytes. (D) Portal inflammation with characteristic lymphocyte-mediated bile duct damage. |
Stainable iron was present in 11 specimens from nine children (22%) and consisted of mild to moderate reticuloendothelial (all specimens), hepatocellular (7 of 11 specimens), and bile ductular (1 of 11 specimens) iron deposition. In seven of these nine children, there was a predisposing clinical history for hemosiderosis including multiple transfusions for thalassemia (2 patients), bone marrow transplantation (2 patients), blood transfusions and parenteral iron for other clinical indications (2 patients), and hemodialysis for end-stage renal disease in a child with a family history of hemochromatosis (1 patient). In the remaining 2 patients, 1 with mild reticuloendothelial iron and 1 with mild reticuloendothelial, hepatocellular, and bile ductular iron, there was no predisposing factor for increased iron stores. Interestingly, both of these patients had perinatally acquired Hepatitis C infection with long-standing disease (13.4 and 16.4 years, respectively) and cirrhosis at the time of initial biopsy.
Other histopathological features included endothelialitis and occasional portal macrophages. Minimal to mild portal and/or central vein endothelialitis was present in 40% of cases, and was characterized by attachment of lymphocytes to the luminal endothelial surface or lymphocytic infiltration between the endothelium and the basal lamina. Histologically recognizable damage to the endothelium was only rarely seen. Occasional pigmented portal macrophages containing periodic acid-Schiff-positive, diastase-resistant cytoplasmic material were present in 10% of cases.
Necroinflammatory Activity (Histological Grade). The histological activity index or histological grade of the disease in each case was determined using three different widely used schemes for evaluation of the necroinflammatory activity.28-30 All three different grading schemes had a positive correlation with the clinical activity at the time of biopsy as determined by serum ALT levels when available (Fig. 2). The most significant association was between ALT levels and the METAVIR histological activity index (Kruskal-Wallis test, P= .005), compared with the Scheuer index (P = .015) and the Knodell index (P = .110). There was no significant association between any of the other clinical parameters listed in table 1 and histological grade in this series.
| Fig. 2. Serum ALT levels versus histological activity index (histological grade) for three different grading schemes. P values are for the Kruskal-Wallis test with the null hypothesis that biopsies grouped by each histological activity index come from patients with the same distribution of serum ALTs. Based on the above analysis, the METAVIR grading scheme had the most significant association with serum ALT levels, followed by the Scheuer and Knodell schemes, respectively. |
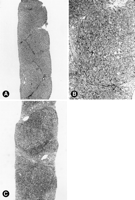
Fibrosis (Histological Stage). Portal fibrosis was present in 78% of the specimens, including fibrous portal expansion (26% of cases), portal-portal and/or portal-central bridging (22% of cases), bridging with architectural distortion (22% of cases), and cirrhosis (8% of cases) (table 2, Fig. 3). Significant fibrosis (bridging fibrosis, bridging with architectural distortion, or cirrhosis) was present in 23 of 40 children (58%). In 2 of these 23 children, bridging fibrosis with architectural distortion was first noted in the posttreatment biopsy, while the pretreatment biopsy showed only portal fibrosis. There was an association between the extent of fibrosis and age (P = .032), and extent of fibrosis and duration of infection (P = .046) as assessed by the Kruskal-Wallis test. However, bridging fibrosis with distortion developed in less than 3 years and as early as 1 year postinfection in 3 of 24 patients in whom the duration of infection was known. Of note, all 3 of these patients acquired Hepatitis C infection by intravenous immunoglobulins. Furthermore, all 3 patients with cirrhosis had stainable iron, including 2 cases with mild reticuloendothelial, and 1 with mild reticuloendothelial, hepatocellular, and bile ductular. One of the 2 patients with reticuloendothelial stainable iron was a bone marrow transplant patient with a history of multiple blood transfusions, but there was no predisposing clinical factor for iron storage in the other 2.
| Fig. 3. (A) Delicate portal fibrosis with portal-to-portal bridging and mild distortion of lobular architecture. Inflammation and necroinflammatory activity are inconspicuous. (B) Higher magnification of portal-to-portal bridging fibrosis, minimal ballooning of hepatocytes, and slight inflammation. (C) Established micro/macronodular cirrhosis. |
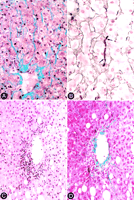
Centrilobular pericellular fibrosis, which was not included to determine the histological stage, was present in 52% of the biopsies (table 2), and consisted of delicate, wire-like bands of collagen radiating from the terminal hepatic venule along the space of Disse (Fig. 4A). The involvement in any given biopsy was patchy, and not all central veins were equally involved. Similar bands of mature collagen were also present focally within the lobules and were particularly evident with the reticulin stain (Fig. 4B). In one biopsy, prominent centrilobular necroinflammatory activity was multifocally present, and was accompanied by a mild pericellular fibrosis (Fig. 4C and 4D). There was no statistical association between any of the clinical variables listed in table 1, and the presence of pericentral pericellular fibrosis in this series.
| Fig. 4. (A) Pericentral pericellular fibrosis characterized by wire-like bands of collagen radiating from the terminal hepatic venule along the space of Disse (Masson trichrome stain). (B) Reticulin stain highlights a dense band of mature collagen filling up the space of Disse (between arrows) and compressing adjacent hepatocytes. (C) Hematoxylin-eosin stain demonstrating centrilobular necroinflammatory activity and endothelialitis (immunohistochemical stain for cytokeratins [monoclonal antibody clone AE1] specific for bile duct epithelium was negative in this area). (D) Trichrome stain of the same field shown in (C) demonstrates dnds of collagen extending from the venule into the lobule along the space of Disse. elicate ba |
DISCUSSION
The histopathological characteristics of chronic HCV infection in adults have been the subject of many recent articles.1-9 Nevertheless, characteristic features of chronic Hepatitis C in children are rarely described. Recently, Kage et al. reported liver histopathology in chronic Hepatitis C in 109 Japanese children with an average age of 6.2 years.27 Kage et al. found that chronic Hepatitis C in children presents with the same histological lesions as in adults (such as lymphoid aggregates, sinusoidal lymphocytosis, and steatosis), but concluded that these changes were generally milder in children. The mean duration of infection in these children was relatively short (3.7 years for 85% in whom the duration of disease was known), and the virus genotype was not reported.
We report the liver histopathology in 40 children with chronic HCV infection followed at a single North American institution. The children had a mean age of 11.4 years, with an average duration of infection of 6.8 years in 24 patients in whom the onset of infection could be reliably dated. The characteristic histopathological lesions of chronic Hepatitis C were present in these children with approximately the same frequency as seen in adults (table 2, Fig. 1). Sinusoidal lymphocytosis was the most common feature, present in 74% of the specimens, compared with 26% to 78% reported in adults.1,5,7 Steatosis has been reported in 54% to 72% of adult cases,.1,5,7 and was present in 50% of the specimens in our series. Portal lymphoid aggregates and/or follicles were present in 48% of our specimens, compared with 49% to 78% in adults..1,5,7 Finally, bile duct epithelial damage that has been noted in 22% to 90% of adult specimens.1,5,7 was present in 28% of our cases. Minimal hepatocellular damage was characteristic in all cases of this series, and in our opinion, it had a diagnostic value helping to distinguish Hepatitis C from other types of chronic hepatitis, particularly autoimmune hepatitis in which hepatocellular damage is more pronounced.
A prominent histopathological feature not previously described in the context of chronic Hepatitis C infection was pericellular fibrosis that was typically seen around the central veins (Fig. 4A), and occasionally within the lobules away from the central veins (Fig. 4B). The fibrosis was subtle and focal, but readily apparent on trichrome and reticulin stains as wire-like bands of collagen within the space of Disse. Centrilobular pericellular fibrosis was present in 52% of specimens![]() essentially as common as steatosis (50%) and portal lymphoid aggregates/follicles (48%). Although the pathogenesis of centrilobular pericellular fibrosis can only be speculated at this point, its association with pericentral necroinflammatory activity in one case (Fig. 4C and 4D) raises the possibility of direct hepatocellular damage in this region as a possible fibrogenic mechanism. This lesion could also represent a sequela of a previous episode of acute hepatitis. Whether the novelty of pericellular fibrosis in our series is related to its unique presence in children, or simply because it has been previously overlooked in adult series, requires additional studies.
essentially as common as steatosis (50%) and portal lymphoid aggregates/follicles (48%). Although the pathogenesis of centrilobular pericellular fibrosis can only be speculated at this point, its association with pericentral necroinflammatory activity in one case (Fig. 4C and 4D) raises the possibility of direct hepatocellular damage in this region as a possible fibrogenic mechanism. This lesion could also represent a sequela of a previous episode of acute hepatitis. Whether the novelty of pericellular fibrosis in our series is related to its unique presence in children, or simply because it has been previously overlooked in adult series, requires additional studies.
Recently, Kage et al. reported the histopathology of chronic Hepatitis C in 109 Japanese children.27 The average histological activity based on the Scheuer grading scheme reported in the series of Kage et al. is comparable with our series (3.8 vs. 3.6); however, there is a significant difference in the stage of fibrosis between the two groups. Kage et al. found no cases of cirrhosis and reported only a 3.6% prevalence of bridging fibrosis with architectural distortion. This compares with an 8% prevalence of cirrhosis and a 22% rate of bridging fibrosis with distortion in our series. Part of this difference may be a result of a slightly longer duration of infection in our series. There may also be an association between the virus genotype and fibrosis in chronic Hepatitis C.20-26 The virus genotype was not reported by Kage et al.,27 but based on genotype data in another Japanese pediatric cohort,17 and given the essential absence of type 1a virus and a relatively larger proportion of type 2 virus in Japan,18,19 the possibility of histopathological differences based on genotype cannot be excluded.
Inui et al. summarized the histological grade and stage of 31 biopsies in 25 Japanese children with transfusion-associated chronic Hepatitis C.16 The pathological description of the cases in that report is limited, but from a graphical summary of the biopsy findings, it can be inferred that 36% of the biopsies showed bridging fibrosis (Knodell stage 3) and 10% showed cirrhosis (Knodell stage 4). The combined prevalence of bridging fibrosis and cirrhosis in that report (46%) is slightly smaller than we have seen (52% of patients). However, the children in the report by Inui et al. had a high preponderance of multiple transfusions (with unknown virus genotype), were on average younger than those in the present report (6 vs. 11 years of age), and had a shorter duration of infection (3.6 vs. 6.8 years on average).16
Matsuoka et al. reported the histological activity of 13 Japanese children with transfusion-associated chronic Hepatitis C infection.17 These children were on average 3.1 years old at the time of transfusion, and all had more than 2 years of clinical chronic hepatitis, but the duration of infection to biopsy was not reported. Matsuoka et al. generally described a mild active hepatitis (average Knodell activity index of 3.7 ± 2.1) with no description of the degree of fibrosis.17 The virus genotype in that study, however, consisted of 75% type 1b, 15% types 2 and 3 each, and no type 1a cases, which is somewhat different from our series of 60% infection with type 1a and 32% type 1b virus.
Bortolotti et al. obtained liver biopsy at presentation of chronic Hepatitis C in 28 Italian and Spanish children (mean age of 4.4 years), and reported chronic active hepatitis in approximately 30%, chronic persistent hepatitis in approximately 40%, and chronic lobular hepatitis or nonspecific reactive hepatitis in approximately 30%.13 Follow-up biopsies were available in 40 patients in the same cohort. Although this study was admittedly limited by a “lack of uniform revision of liver biopsies,” it suggested that severe active hepatitis and cirrhosis were infrequently associated with chronic HCV infection in childhood and adolescence.13
In another European study of thalassemic children, Lai et al. reported the pathology of chronic Hepatitis C infection in 43 children, including 15 (35%) with chronic persistent hepatitis, 22 (51%) with chronic active hepatitis, and 6 (14%) with cirrhosis.12 Little additional information is available about the liver pathology in chronic Hepatitis C in this and few other reports.11,12,14,15
The average histological activity index in our experience was relatively mild (Fig. 2), and the associated hepatocellular damage was small given the extent of the lobular inflammation. In our hands, the METAVIR histological activity index was more strongly associated with the clinical activity as determined by serum ALT levels at the time of biopsy.
In spite of a relatively mild degree of necroinflammatory activity, we observed significant portal fibrosis in 23 of 40 children (58%) (table 2, Fig. 3). Overall, portal fibrosis was present in 78% of the specimens, including fibrous portal expansion (26% of cases), portal-portal and/or portal-central bridging (22% of cases), bridging with architectural distortion (22% of cases), and cirrhosis (8% of cases) (table 2). The extent of portal fibrosis was positively correlated with age and duration of infection, although in the cohort of patients infected by intravenous immunoglobulins, significant portal fibrosis was seen in less than 3 years, and as early as 1 year postinfection, suggesting that these children may have received a large virus load at the time of infection. Also of note, in 2 of the 23 children with bridging or more severe fibrosis, bridging fibrosis with architectural distortion was first noted in the posttreatment biopsy, while the pretreatment biopsy showed only portal fibrous expansion. In both of these children, there was near-complete resolution of the necroinflammatory activity on the posttreatment biopsies, and neither had detectable viral RNA in the serum by polymerase chain reaction. The more pronounced portal fibrosis in the posttreatment biopsies may be explained by sampling artifact, because fibrosis may not be uniform throughout the liver. However, the possibility that progression of fibrosis in chronic Hepatitis C infection may continue in spite of response to treatment cannot be ruled out.
The role of hepatic iron stores in hepatic damage caused by chronic HCV infection is not clear. Overall, positive iron staining has been reported in 7% to 63% of patients,31-34 compared with 22% in our series. Although iron-mediated hepatocellular damage may be a pathogenic mechanism in Hepatitis C infection,31 previous studies have indicated no association between hepatic iron stores and fibrosis,32 and both present and absent correlation with the histological activity index.32,34 The number of patients with stainable iron in our series is too small for a statistical analysis of the relationship between viral infection, iron overload, activity, and fibrosis. Nonetheless, all three children with cirrhosis in our series had mild stainable iron, and only one had a predisposing clinical factor (multiple transfusions) for iron overload. On the other hand, both of these children had acquired infection perinatally, and had long-standing disease at the time of biopsy. These data preclude a definitive assessment of the relationship between chronic Hepatitis C infection, iron overload, and fibrosis.
In summary, we have presented the liver histopathology in 40 children with chronic Hepatitis C infection followed at a single institution over the past decade. Our findings indicate that the characteristic histopathological lesions of chronic Hepatitis C infection, including sinusoidal lymphocytosis, steatosis, portal lymphoid aggregates/follicles, and bile duct epithelial damage, occur with relatively the same frequency in children as has been reported in adults. Pericentral and lobular pericellular fibrosis, which has not been previously reported in the context of chronic Hepatitis C infection, was also a prominent feature in our series, occurring with a similar frequency as steatosis or lymphoid aggregates/follicles. The overall progression of fibrosis seen with increasing age and duration of infection underscores that the natural history of chronic HCV infection in childhood is not benign, and in some instances, may lead to significant subsequent morbidity.
Abbreviations
Abbreviations: HCV, Hepatitis C virus; ALT, alanine transaminase.
Footnotes
Received May 7, 1998; accepted June 25, 1998.
Dr. Nelson’s current address is: Children’s Memorial Medical Center, Chicago, IL.
Address reprint requests to: Antonio Perez-Atayde, M.D., Department of Pathology-Bader 1, Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115. Fax: (617) 731-0954.
Copyright © 1998 by the American Association for the Study of Liver Diseases.