HEPATOLOGY, August 1998, p. 341-346, Vol. 28, No. 2
Sequential Versus Concomitant Administration of Ribavirin and Interferon Alfa-n3 in Patients With Chronic Hepatitis C Not Responding to Interferon Alone: Results of a Randomized, Controlled Trial
Raffaello Sostegni1, Valeria Ghisetti2, Fabrizia Pittaluga2, Giovanna Marchiaro2, Giuseppe Rocca1, Elisabetta Borghesio1, Mario Rizzetto1, and Giorgio Saracco1
From the 1 Dipartimento di Gastroenterologia, Ospedale Molinette, Università di Torino, and the 2 Laboratorio di Microbiologia, Ospedale Molinette, Torino, Italy.
ABSTRACT
We conducted a three-arm, randomized trial in 96 patients with chronic hepatitis C who did not respond to interferon alfa to compare treatments. Group 1 (33 patients) received ribavirin alone (1,000 mg/daily for 6 months) followed by interferon alfa n-3 alone (3 MU thrice weekly for 6 months); group 2 (33 patients) received ribavirin plus interferon alfa n-3 for 6 months at the above doses; and group 3 (30 patients) received interferon alfa n-3 alone (3 MU thrice weekly for 6 months). At the end of treatment, 3 patients (10%) in group 1, 13 (41%) in group 2, and 5 (17%) in group 3 had normal alanine transaminase (ALT) levels (group 2 vs. groups 1 and 3, P = .008). After 6 months of follow-up, only 4 patients (12.5%) in group 2 still had normal ALT values (P = .03). At the end of therapy, hepatitis C virus (HCV) RNA was no longer detectable by polymerase chain reaction in 4 (13%), 9 (27%), and 2 (7%) patients, respectively, in groups 1, 2, and 3 (P = NS). Six months posttherapy, only 5 (15%) patients in group 2 were still HCV RNA negative (P = .02). At the time of follow-up liver biopsy, performed 6 months after the end of treatment, a significant improvement of the necroinflammatory scores was observed among group 2 patients (P = .01) but not in the other two groups. Side effects reflected the profile of each drug as monotherapy; mild hemolytic anemia was the most frequent side effect caused by ribavirin. In conclusion, concomitant administration of ribavirin and interferon alfa n-3 was significantly superior to the sequential schedule or interferon alfa n-3 monotherapy in inducing a sustained response in patients with chronic hepatitis C who had not responded to interferon alone. However, combination therapy at the dose and duration adopted in this study is capable of modifying the natural course of the disease in only a minority of these patients. (HEPATOLOGY 1998;28:341-346.)
INTRODUCTION
Interferon (IFN) monotherapy induces a permanent remission in a minority of treated patients with chronic hepatitis C.1 Patients in whom IFN monotherapy is not effective do not respond to either initial or repeat IFN therapy. Their alanine transaminase (ALT) levels do not normalize, even during IFN administration.2,3Thus, retreatment of nonresponders with IFN alone generally is considered ineffective.4 Pilot studies5,6 using ribavirin (a broad-spectrum antiviral drug given by mouth) with IFN have reported sustained responses in approximately 40% of patients who had a relapse or no response to IFN alone. Further studies have shown that the response to combination therapy is significantly different between patients who had no response to IFN and those who had a response while undergoing therapy but then relapsed; with combination therapy a sustained response is observed in most relapsers but in only few nonresponders.7,8 A recent meta-analysis9 has shown that the estimated probability of sustained response after IFN-ribavirin combination therapy is approximately 16% for previous IFN nonresponders; the standard schedule currently proposed is concomitant administration of 1,000/1,200 mg of ribavirin orally each day and 3,000,000 IU (3 MU) of IFN thrice weekly for 6 months. The aim of this trial was to establish the efficacy and tolerability of IFN-ribavirin combination therapy administered according to two different temporal schedules (ribavirin monotherapy followed by IFN vs. concomitant administration of ribavirin and IFN) compared with IFN therapy alone in patients with chronic hepatitis C that did not respond to previous IFN therapy.
Pretherapy features were also evaluated by univariate analysis to determine whether associations exist between baseline variables and a sustained response.
PATIENTS AND METHODS
Ninety-six patients with chronic hepatitis C that did not respond to previous courses of recombinant IFN-![]() or lymphoblastoid IFN administered at a minimum dose of 9 MU/wk for a minimum of 12 weeks were enrolled in an Italian randomized, controlled trial. All patients were considered nonresponders because they did not have normal ALT levels and clearance of viremia at the end of a single course of IFN therapy. Patients were eligible if they had all the inclusion criteria: age >18 years and <65 years; positive results for antibody to hepatitis C virus (HCV); positive results for HCV RNA by polymerase chain reaction (PCR); chronic hepatitis C at liver biopsy; previous nonresponse to IFN-
or lymphoblastoid IFN administered at a minimum dose of 9 MU/wk for a minimum of 12 weeks were enrolled in an Italian randomized, controlled trial. All patients were considered nonresponders because they did not have normal ALT levels and clearance of viremia at the end of a single course of IFN therapy. Patients were eligible if they had all the inclusion criteria: age >18 years and <65 years; positive results for antibody to hepatitis C virus (HCV); positive results for HCV RNA by polymerase chain reaction (PCR); chronic hepatitis C at liver biopsy; previous nonresponse to IFN-![]() alone (9 MU/wk for 12 weeks at least); and evidence of elevated ALT levels (at least 1.5 times upper limit of normal; range, 0-40 IU). The exclusion criteria were relapses after a previous course of IFN
alone (9 MU/wk for 12 weeks at least); and evidence of elevated ALT levels (at least 1.5 times upper limit of normal; range, 0-40 IU). The exclusion criteria were relapses after a previous course of IFN![]() – alone; positivity for hepatitis B serum antigen; positivity for antibody to human immunodeficiency virus; alcoholic liver disease; hemochromatosis; Wilson’s disease; drug-related liver disease; autoimmune hepatitis; inadequate hemoglobin level (<10 g/dL), platelet count (<70,000/mm3), white blood cell count (<3,000/mm3), or granulocyte count (<1,500/mm3); decompensated cirrhosis; intravenous drug abuse; abnormal serum uric acid level; presence of concomitant significant medical illness; history of hemolytic anemia; 1-antitrypsin deficiency; obesity-induced liver disease; and hemophilia. Patients were randomly assigned by computer coding to one of the following study groups: group 1 (33 patients), 1,000 mg/daily ribavirin for 6 months followed by 3 MU natural human leukocyte IFN-
– alone; positivity for hepatitis B serum antigen; positivity for antibody to human immunodeficiency virus; alcoholic liver disease; hemochromatosis; Wilson’s disease; drug-related liver disease; autoimmune hepatitis; inadequate hemoglobin level (<10 g/dL), platelet count (<70,000/mm3), white blood cell count (<3,000/mm3), or granulocyte count (<1,500/mm3); decompensated cirrhosis; intravenous drug abuse; abnormal serum uric acid level; presence of concomitant significant medical illness; history of hemolytic anemia; 1-antitrypsin deficiency; obesity-induced liver disease; and hemophilia. Patients were randomly assigned by computer coding to one of the following study groups: group 1 (33 patients), 1,000 mg/daily ribavirin for 6 months followed by 3 MU natural human leukocyte IFN-![]() (IFN-n3, Alfaferone; Alfa-Wasserman, Bologna, Italy) thrice weekly for an additional 6 months; group 2 (33 patients), 1,000 mg ribavirin daily plus 3 MU IFN-n3 thrice weekly for 6 months; and group 3 (30 patients), 3 MU IFN-n3 thrice weekly for 6 months. In groups 1 and 2, ribavirin was supplied as 200-mg capsules given orally in two divided doses of 1,000 mg daily. Patients were seen every month during therapy and follow-up. At each visit, blood was drawn for routine hematologic and biochemical tests, and aliquots of serum were stored at
(IFN-n3, Alfaferone; Alfa-Wasserman, Bologna, Italy) thrice weekly for an additional 6 months; group 2 (33 patients), 1,000 mg ribavirin daily plus 3 MU IFN-n3 thrice weekly for 6 months; and group 3 (30 patients), 3 MU IFN-n3 thrice weekly for 6 months. In groups 1 and 2, ribavirin was supplied as 200-mg capsules given orally in two divided doses of 1,000 mg daily. Patients were seen every month during therapy and follow-up. At each visit, blood was drawn for routine hematologic and biochemical tests, and aliquots of serum were stored at ![]() 80°C immediately after collection.
80°C immediately after collection.
All details of the study were approved by the ethical committee, and patients gave written informed consent for inclusion. Efficacy was assessed by monitoring of serum ALT levels and plasma HCV RNA levels. Safety was assessed by collection of adverse event data and by periodic hematology and biochemistry tests. The biochemical response to IFN-n3 was defined as complete when ALT levels were normal at the end of therapy. The response was considered sustained if ALT values were normal during the 6-month follow-up. A relapse was defined as a return to abnormal ALT levels after a complete response within the 6-month posttherapy period. All the patients underwent liver biopsies within the 6 months before inclusion into the study; a second liver biopsy was proposed to each patient at the end of follow-up. Each liver biopsy was reviewed blindly by two pathologists according to the Ishak’s scoring system.10
Virology
Serum for HCV RNA levels was measured at the start of treatment, at the end of therapy, and at the end of follow-up; in group 1, an additional HCV RNA quantitation was performed at the end of ribavirin administration. HCV RNA was quantified by branched DNA signal amplification assay (Quantiplex, version 2.0) according to the manufacturer’s instructions (Chiron Corporation, Emeryville, CA). This assay has a sensitivity of 2 × 105 HCV genome equivalents/mL (genEq/mL); serum samples below this cut-off were evaluated for HCV RNA by a sensitive nested PCR. HCV RNA was extracted from 50 µL of serum, reverse transcribed, and amplified as previously described.11 The sensitivity of this assay was 50 genEq/mL of serum. HCV genotyping was carried out using a probe-specific hybridization assay (Line probe assay, LIPA HCV; Innogenetics, Ghent, Belgium).
Statistical Analysis
Dichotomous variables were compared using the ![]() 2 test, and quantitative variables were compared with Student’s t test. To determine the strength of association between pretreatment selected variables and sustained response, a logistic regression model was used.
2 test, and quantitative variables were compared with Student’s t test. To determine the strength of association between pretreatment selected variables and sustained response, a logistic regression model was used.
RESULTS
Two patients in group 1 did not receive therapy; therefore, the intent population comprised 94 patients. No significant differences were found between the groups regarding age, sex, mean baseline ALT values, liver histology, source of infection, viremia, or genotypes table 1).
| View This table |
table 1. Patients Characteristics |
.gif) |
Fig. 1. Mean ALT levels in the 3 groups. ( |
At the end of treatment, mean ALT levels were significantly reduced among patients in group 2 compared with those in the other two groups (P = .01); this difference was still present at the end of follow-up (P = .007).
Virological Analysis
The mean quantitative levels of HCV RNA detected in the three groups during therapy and follow-up are reported in Fig. 2; in each group, an insignificant reduction in viremia was observed at the end of treatment compared with baseline levels (group 1, 4.1 vs. 4.9 × 106; group 2, 1.3 vs. 3.1 × 106; group 3, 2.4 vs. 5.1 × 106 genEq/mL), but the mean HCV RNA levels returned to pretherapy values at the end of follow-up in all groups. No significant difference in mean viremic levels among the three groups was observed at the end of therapy and follow-up. In group 1, the decrease in viremia was more pronounced at the end of ribavirin administration than that measured at the end of the IFN-n3 course; this effect was not limited to patients with favorable predictive factors (low viremia, non-1 genotype, mild histological picture) but was seen in almost all the patients.
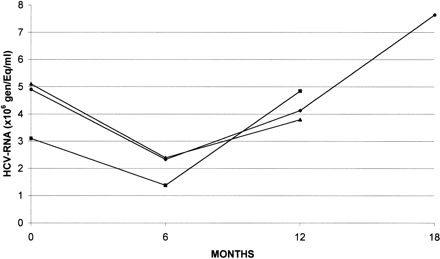 |
Fig. 2. Mean quantitative levels of HCV RNA during treatment and follow-up.( ( |
At the end of treatment, HCV-RNA was no more detectable by PCR in 4 (13%), 9 (27%), and 2 (7%) patients, respectively, in groups 1, 2, and 3 (P = NS). Six months later, only 5 (15%) patients in group 2 were still HCV RNA-negative (P = .02). Four of these 5 patients also had normal ALT levels.
Histology
The mean necroinflammatory score and the mean staging score were similar in the three groups at the start of therapy (table 1). Of the 96 patients included in this study, 28 agreed to undergo a second liver biopsy 6 months after the end of therapy: 8 in group 1, 12 in group 2, and 8 in group 3 (table 2). All 4 patients in group 2 who had sustained responses underwent second biopsies.
| View This table | table 2. Histological Changes |
Factors Predictive of Sustained Response
The 4 patients with sustained responses and the 29 with no response or relapse in group 2 were stratified according to sex, age, mean baseline ALT levels, source of infection, viremia, genotype, and histology. Because of the limited number of patients considered, only a univariate logistic regression analysis was performed to correlate these baseline characteristics with the long-term outcome of IFN therapy. Results are reported in table 3 Low levels of HCV RNA and history of blood transfusions were the only significant factors influencing sustained response.
| View This table | table 3. Results of Univariate Logistic Regression Analysis Correlating Initial Features With Long-Term Outcome of Patients in Group 2 |
Overall, 11 (11%) patients discontinued treatment early because of side effects: 5 in group 1, 4 in group 2, and 2 in group 3. Three of the 5 patients who dropped out in group 1 left the study during ribavirin administration: 1 for severe pruritus, 1 for abdominal pain, and 1 for severe hemolytic anemia (7 g/dL); the other 2 patients discontinued IFN-n3 treatment because of severe mental depression. In group 2, 2 patients discontinued therapy for headache, 1 for severe mental depression, and 1 for an ischemic cardiac episode probably triggered by a mild decrease in hemoglobin (10 g/dL). Finally, 2 patients in group 1 discontinued IFN-n3 therapy for headache and myalgias. These symptoms promptly reversed after therapy was discontinued.
There were no statistically significant differences among groups regarding frequency of side effects, with the exception of anemia; the mean ± SD decreases in hemoglobin in groups 1 and 2 were 2.3 ± 1.11 and 2.5 ± 1.14 g/dL, respectively. Because of hemolytic anemia, a dose reduction (usually 20% of the total dose of ribavirin) was necessary in 6 (24%) patients in group 1 and 8 (27.5%) patients in group 2. No change in IFN-n3 dosage was observed. The pattern of mean hemoglobin levels and reticulocyte counts of the three groups is shown in Fig. 3. The decrease in hemoglobin was accompanied by a parallel decrease in red blood cell count, but both of the biochemical abnormalities reversed after suspension of ribavirin therapy.
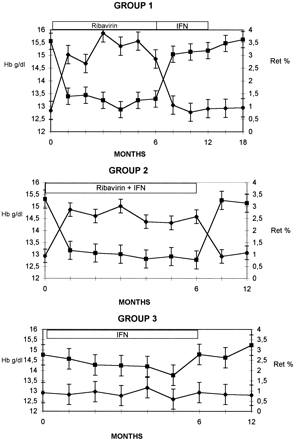 |
Fig. 3. Hemoglobin levels and reticulocyte counts in the 3 groups (means ± SD). |
DISCUSSION
Retreatment with IFN for patients who did not benefit from a first therapeutic cycle with the cytokine has been attempted in several studies,with disappointing results. There has been no significant response to either initial or repeat IFN administration; in particular, increasing the dose or the duration did not confer a benefit. To date, the most promising alternative therapy for these patients is the nucleoside analog ribavirin; although this drug induces only a transient beneficial effect when given as monotherapy,12-14 it seems to increase in effectiveness when associated with IFN.4 There is evidence that the response to combination therapy is significantly different between nonresponders, i.e., those who did not have a response during therapy, and relapsers, i.e., those who responded during therapy but then rebounded after treatment withdrawal. According to a recent meta-analysis,9 approximately 50% of those who relapse after a first IFN course have a sustained response with combination therapy, compared with only 19% of nonresponders; in recent studies,15,16 combination therapy was of no advantage compared with IFN alone for retreatment of nonresponders. Our data confirm that only a minority (12.5%) of IFN-resistant patients have a sustained response when treated with the combination of ribavirin and IFN, indicating that sequential administration of the two drugs is ineffective and has no impact in reducing the frequency of side effects. Although modest, these results represent some therapeutic gain for patients who are likely to have a progressive clinical course. Patients treated with simultaneous combination therapy had significantly lower ALT levels during the follow-up than those in the other two groups. Mean HCV RNA values were not different among the three groups at the end of follow-up, but all 4 sustained responders in group 2 were no longer viremic, in indication that they were likely to have attained permanent viral eradication. The reduction of viremia in group 1 at the end of the 6-month course with ribavirin alone was not significant; a similar finding was reported by other authors. Di Bisceglie et al.13 reported a decrease in HCV RNA titers during therapy and for several months afterward; a viremic decrement at the end of therapy was found also by Dusheiko et al.14 whereas in a Swedish study,12 mean HCV RNA levels decreased from 4.1 × 106 to 3.4 × 106genEq/mL at cessation of therapy. The surprising progressive increase in viremia observed in group 1 during IFN treatment after ribavirin withdrawal suggests that ribavirin may play a detrimental role on the action of IFN, possibly by enhancing the selection of viral strains resistant to IFN. The mechanism whereby ribavirin is able to potentiate the effect of IFN when administered given in combination is still unknown; the hypothesis that the drug acts by eliminating the intracellular reservoirs of HCV not accessible to IFN17 was not confirmed.18The hypothesis of an immunomodulatory effect of ribavirin19 needs further evidence.
Ribavirin combined with IFN was well tolerated at a dose of 1,000 mg/d for 6 months. Only 4 patients (3 in group 1 and 1 in group 2) discontinued therapy early because of side effects probably related to the ribavirin administration. The drug was also safe in the consistent proportion of cirrhotic patients who are less tolerant to a wide variety of therapeutic agents.
In conclusion, with the schedule used in this study, the natural history of HCV-positive nonresponders to IFN was not significantly modified. Further trials with higher doses and/or longer duration of the combination of ribavirin and IFN are needed to improve efficacy.
Footnotes
Abbreviations: IFN, interferon; ALT, alanine transaminase; HCV, hepatitis C virus; PCR, polymerase chain reaction.
Received January 7, 1998; accepted April 6, 1998.
Address reprint requests to: Giorgio Saracco, M.D., Department of Gastroenterology, Molinette Hospital, Corso Bramante 88 10126 Torino, Italy. Fax : 39-11-6634213.
Copyright © 1998 by the American Association for the Study of Liver Diseases.




