Harvoni – A Complete Guide


Below you will find the ultimate guide to Harvoni, a prescription medication to treat the Hepatitis C virus (HCV) that was approved by the U.S. Food and Drug Administration in 2014. While there are multiple HCV treatment options available in the U.S., Harvoni was the first once-daily pill that didn’t require interferon or ribavirin.
What Is Harvoni?
Harvoni is an HCV treatment that comes in pill form consisting of 2 HCV-fighting drugs (sofosbuvir and ledipasvir).
It is approved in the United States for patients over 18 years old who are HIV-negative and HIV-positive with HCV genotypes 1, 4, 5, and 6. It is also approved for those with HCV genotype 1 who have advanced (decompensated) cirrhosis as well as transplant recipients with HCV genotype 1 or 4.
It is taken once per day, with or without food, between 8 and 24 weeks. The length of treatment is dependent on the patient’s HCV treatment history, if they have cirrhosis, and how much HCV is in the bloodstream.
Some patients will need to take an additional drug, called ribavirin (RBV), twice a day with Harvoni.
Generally, Harvoni has a success rate between 94 and 100% for patients with genotype 1.
Genotype 1 Adults Without Cirrhosis
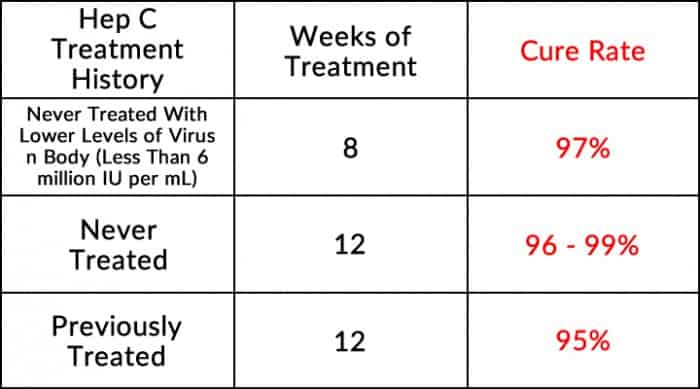
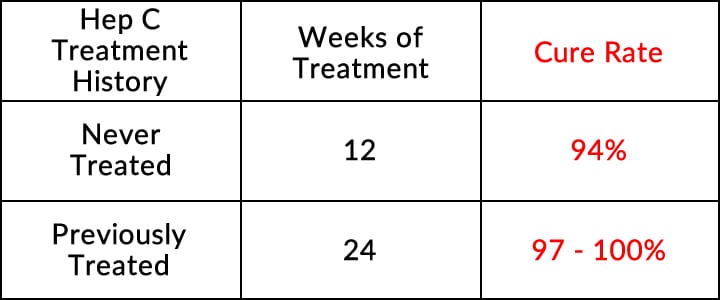
Genotype 1 Adults With Compensated Cirrhosis
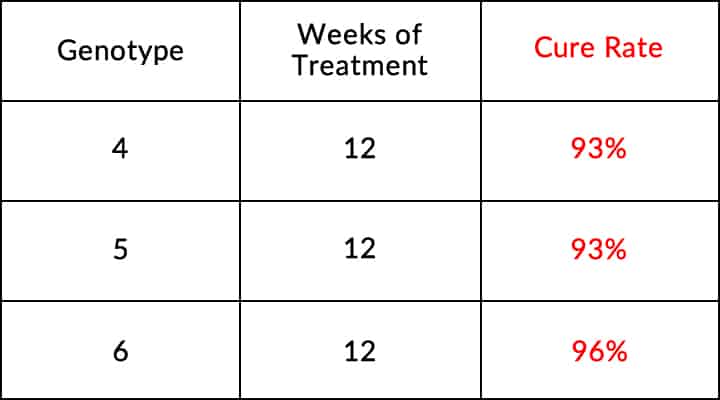
Adults With Genotypes 4, 5, or 6 With or Without Cirrhosis
Side Effects of Harvoni
Common side effects are usually mild cases of fatigue, headache, nausea, diarrhea, and insomnia. Some patients have reported skin swelling, rash, or blisters.
What Drugs Should Not Be Taken With Harvoni?
Harvoni should not be combined with amiodarone as it can cause life-threatening heart problems.
For those who must take amiodarone, intensive heart monitoring in a hospital is recommended for 48 hours after starting Harvoni, and daily monitoring for at least 2 weeks afterwards.
Talk with your physician prior to starting or stopping any medications, supplements, or herbal remedies. Some medications should be alternated, stopped or avoided while using Harvoni.
You should not take Harvoni if you’re taking any of the following drugs:
- Antivirals like Aptivus (tipranavir) and Olysio (simeprevir)
- Cordarone, Pacerone, or Nexterone (amiodarone)
- Crestor (rosuvastatin)
- Dilantin and Phenytek (phenytoin)
- Equetro, Carbarol, or Tegretol (carbamazepine)
- Lanoxin (digoxin)
- Rifadin or Rimactine (rifampin)
- The herbal supplement St. John’s wort
Other drugs that may have serious interactions with Harvoni include:
- Antacids, like aluminum hydroxide, Tums (calcium carbonate), and sodium bicarbonate
- Axid (nizatidine), Pepcid (famotidine), Tagamet (cimetidine), and Zantac (ranitidine)
- Milk of Magnesia (magnesium hydroxide)
- Proton pump inhibitors (PPIs) like Dexilant (dexlansoprazole), Nexium(esomeprazole), Prilosec (omeprazole), and Protonix (pantoprazole)
- Tagamet (cimetidine)
- Videx and Videdx EC (didanosine)
Can Hep C Come Back After Taking Harvoni?
Once treatment is complete, patients have a high chance of being cured of HCV. But they will still need to take certain precautions. 12 weeks after completing treatment, patients need to take a PCR viral detection test to confirm if they are cured. They will not know if they are cured till they take this test.
Once cleared of HCV, patients still aren’t immune from catching it again. It’s best to lower the risk by avoiding blood-to-blood contact with other people, practicing safe sex, and not sharing needles.
If cured, you still may have existing liver damage. If you have cirrhosis, you still have a risk of liver cancer, even after being cured of HCV. If you have serious liver damage, it is advised to continue seeing a liver specialist who can monitor the issue.
In the rare case that Harvoni did not cure you, it could be due to various reasons such as genetics, the virus mutating, or you missed taking pills during treatment. When this happens, you can consider extending your treatment or trying a different treatment.
How Much Does Harvoni Cost?
A 12-week regimen of Harvoni costs $94,500.
Click here to learn more about getting assistance to pay for Harvoni.
To find out if Harvoni is covered by your insurance, you should contact your insurance company directly for information.
Is There a Generic Version of Harvoni?
You can find generic versions of Harvoni in Bangladesh, Egypt, and India at a significantly cheaper price of about $1,000 or less for a 12-week treatment. The Indian version is chemically identical to the brand name version in the United States.
Gilead Sciences, Inc., the U.S. manufacturer of Harvoni recently announced that their newly created subsidiary, Asegua Therapeutics LLC will be producing a generic form of Harvoni that will be available in January of 2019.
At launch, the generic version will be available at a list price of $24,000 for the most common course of therapy.
This authorized generic version is priced to more closely align with discounts that health insurers and government payers receive today. Insurers will have the option of offering either the authorized generics or the branded medication.
2017. “The Harvoni Price Why Does Harvoni Cost so Much.” Hepatitis C Treatment, BSP Corporate, 2017, www.generichepatitiscdrugs.com/harvoni-price/.
“A Simple Treatment Regimen for Hep C.” Official Site | HARVONI® (Ledipasvir 90 Mg/Sofosbuvir 400 Mg) Tablets, Gilead Sciences, Inc., 2018, www.harvoni.com/discover-harvoni/clinical-study-results.
“After Treatment (Hep C) - Post-Treatment.” Hepatitis NSW, 1 Aug. 2018, www.hep.org.au/hep-c-treatment/after-treatment-hep-c/.
“AIDS and HIV Health Center.” WebMD, WebMD, www.webmd.com/hiv-aids/default.htm.
“Amiodarone Oral : Uses, Side Effects, Interactions, Pictures, Warnings & Dosing.” WebMD, WebMD, www.webmd.com/drugs/2/drug-4521/amiodarone-oral/details.
“Gilead Subsidiary to Launch Authorized Generics of Epclusa® (Sofosbuvir/Velpatasvir) and Harvoni® (Ledipasvir/Sofosbuvir) for the Treatment of Chronic Hepatitis C.” Gilead, Gilead Sciences, Inc., 24 Sept. 2018, www.gilead.com/news/press-releases/2018/9/gilead-subsidiary-to-launch-authorized-generics-of-epclusa-sofosbuvirvelpatasvir-and-harvoni-ledipasvirsofosbuvir-for-the-treatment-of-chronic-hepatitis-c.
“Harvoni Fact Sheet.” TB Quick Facts | Treatment Action Group, www.treatmentactiongroup.org/hcv/factsheets/harvoni.
“Hepatitis C.” Mayo Clinic, Mayo Foundation for Medical Education and Research, 6 Mar. 2018, www.mayoclinic.org/diseases-conditions/hepatitis-c/symptoms-causes/syc-20354278.
“Ledipasvir.” DrugBank, www.drugbank.ca/drugs/DB09027.
“Ribavirin.” Treatment Action Group, Nov. 2015, www.treatmentactiongroup.org/hcv/factsheets/ribavirin.
“Sofosbuvir.” DrugBank, www.drugbank.ca/drugs/DB08934.
“WebMD Sexual Conditions Center - Information on STDs, Safe Sex, and Common Sexual Problems.” WebMD, WebMD, www.webmd.com/sexual-conditions/default.htm.
“What Is Hepatitis C?” WebMD, WebMD, www.webmd.com/hepatitis/digestive-diseases-hepatitis-c#1.







