Hepatology, February 1999, p. 590-596, Vol. 29, No. 2
A Prospective, Community-Based Evaluation of Liver Enzymes in Individuals With Hepatitis C After Drug Use
Thomas V. Inglesby1,
From the 1Divisions of Infectious Diseases and 2Gastroenterology, The Johns Hopkins School of Medicine, and the 3Department of Epidemiology, The Johns Hopkins School of Hygiene and Public Health, Baltimore, MD.
ABSTRACT
Serum alanine transaminase (ALT) levels are used to select hepatitis C virus (HCV)-infected patients for treatment and liver biopsy. However, the natural history of these measurements is poorly understood. To examine the hypothesis that ALT levels vary over time in HCV-infected patients, serial serum ALT levels were prospectively measured in a cohort of 1,235 persons with a history of prior illicit drug use. Over 25 months of follow-up, there was a median of four evaluations per patient. ALT values were higher in 1,164 (94%) HCV-infected individuals than in 71 (6%)HCV-uninfected individuals. The remainder of the analysis focusedon these HCV-infected individuals, 647 (62%) of whom had normalALT values at their initial visit. However, 323 (49%) of thesehad at least one elevated ALT over the next 25 months. Of the395 patients whose ALT was initially abnormal, 332 (84%) had at least one normal value over the next 25 months. Overall, among individuals with four or more visits, ALT values were persistently normal in 42%, persistently elevated in 15%, and intermittently elevated in 43%. Because serum ALT levels have high visit-to-visit variability, single assessments should not be used to manage HCV-infected individuals. Further investigation is needed to ascertain the correlation of serial ALT trends with important disease outcomes. (HEPATOLOGY 1999;29:590-596.)
INTRODUCTION
Approximately 4 million persons in the United States have hepatitis C virus (HCV) infection, and an estimated 170 million are infected worldwide.1,2 HCV infection persists in approximately 85% of individuals, who over 20 to 30 years may develop cirrhosis and liver cancer.1 Interferon and interferon alfa-2b combined with ribavirin (Rebetron) are approved by the United States Food and Drug Administration for treatment of HCV infection. Sustained virological responses after 12 months of therapy range from 20% to 40%.3 No test uniformly predicts which individuals will have persistent infection, develop cirrhosis, or respond to therapy, although viral load, HCV genotype, liver histology, and serum transaminases have been studied.4-7
Long-term elevation in serum transaminases, especially alanine transaminase (ALT), was once the principal indication of persistent HCV infection, formerly called non-A, non-B hepatitis. However, up to 30% of HCV infections persist without detectable ALT elevation.8-10 Serum ALT has also been correlated with the degree of histological inflammation in the liver. However, histologically mild disease has been recognized in individuals with elevated ALT values, and histologically advanced disease has been demonstrated in individuals with normal or minimally elevated ALT measurements.11,12 In addition, ALT normalization 8 to 12 weeks after interferon therapy predicts the ultimate response in some, but not all, individuals.13,14
These limitations notwithstanding, serum transaminase measurements are inexpensive, safely obtained, and readily available, and continue to be widely used in the management of HCV infection. For example, a recent NIH Consensus Panel recommended that individuals with normal ALT levels not receive interferon therapy for HCVinfection.15 In addition, in a recent survey of 1,249 gastroenterologists and hepatologists, the result of a single ALT determination dramatically affected the frequency with which liver biopsies were performed.16
Different estimates of the value of liver enzyme testing may derive from limitations of existing studies. Almost all existing studies are based in liver disease clinics, though it is estimated that fewer than 10% of HCV-infected individuals are followed in this setting. In addition to the referral bias of liver disease clinic-based studies, cross-sectional studies cannot evaluate ALT trends, and most prospective studies include few participants. Moreover, many HCV-infected individuals followed in clinical centers have received treatment, making natural history studies impossible. In this investigation, the temporal trends and demographic correlates of liver enzymes were prospectively assessed among a large cohort of almost entirely untreated HCV-infected individuals in the community.
PATIENTS AND METHODS
Population and Study Design. Since 1989, a cohort of former and current injection drug users in the AIDS Linked to the Intravenous Experience (ALIVE) study has been followed semiannually at Johns Hopkins University (Baltimore,MD).17 Using a historical prospective study design, HCV infection in this group was evaluated at enrollment and at subsequent visits using a second-generation enzyme immunoassay (Ortho Diagnostics, Raritan, NJ), as previously described.18,19 At enrollment, the cohort was 81% male, 90% black, 25% human immunodeficiency virus (HIV)-positive, the median age was 34 years, and the median duration of drug use was 13 years.20
Between January 1995 and February 1997, liver enzymes including ALT, aspartate transaminase (AST), and ![]() -glutamyl transferase (GGT) were evaluated at each patient visit. At that time, 1,306 (58.7%) participants in the prospective study were still in follow-up, 567 (25.5%) were lost to follow-up, and 353 (15.9%) were dead. Liver enzyme testing was performed within 24 hours of serum procurement by Quest Diagnostics (Baltimore, MD). Liver enzyme testing was performed on an Olympus 5200 Multichannel chemistry analyzer. The procedure is based on the reference method as recommended by the International Federation of Clinical Chemistry. The quality-control coefficient of variation averaged less than 3%.
-glutamyl transferase (GGT) were evaluated at each patient visit. At that time, 1,306 (58.7%) participants in the prospective study were still in follow-up, 567 (25.5%) were lost to follow-up, and 353 (15.9%) were dead. Liver enzyme testing was performed within 24 hours of serum procurement by Quest Diagnostics (Baltimore, MD). Liver enzyme testing was performed on an Olympus 5200 Multichannel chemistry analyzer. The procedure is based on the reference method as recommended by the International Federation of Clinical Chemistry. The quality-control coefficient of variation averaged less than 3%.
A standardized questionnaire, administered at enrollment and in modified form at each visit, elicited the following patient characteristics: gender, age, race, illicit drug use (including frequency of use, years of use, and specific information regarding method of heroin and cocaine use), methadone use, alcohol use (including information regarding frequency and quantity), and tobacco use. This questionnaire was readministered at each ALIVE study visit during this time period, occurring at least twice for each patient during the study period. HIV testing was also performed at each of these study visits. CD4 measurements were performed in HIV-infected individuals. Serological markers were obtained at baseline for hepatitis Bvirus.
Reference Populations. For reference, ALT levels were also examined for Baltimore-based blood donors and controls. An abnormal ALT was defined as >40 IU/L. The blood donor sample represented 2,493 nonrepeat donations that were HCV-, hepatitis B virus-, HIV-, and rapid plasma reagin-negative. The reference laboratory controls included 2,105 individuals who had ALT measurements performed as part of a full chemistry panel in the same laboratory as study individuals.
Definitions. A normal liver enzyme value was defined according to the range established by Quest Diagnostics and was verified by performing statistical analysis on approximately 3,000 random internal-medicine patients. Extreme values were excluded, and the 95th percentile was used as the upper limit of normal. Normal ranges were verified at the reference laboratory every 6 months and did not change during the course of this investigation. HCV infection was defined by detection of HCV antibody, an inference that was substantiated by HCV-RNA testing. Using second-generation branched-DNA kits (Quantiplex 2.0, Chiron Corporation, Emeryville, CA) HCV RNA was detected on 877 (87.7%) of 1,002 consecutive HCV antibody-positive samples taken at the same visit as the liver enzyme testing.21 However, HCV antibody, not RNA, was used to ascertain infection, because RNA detection can be intermittent over time, and very low levels of viremia may be undetectable with this assay.9Enrollment visit refers to the patient’s first evaluation in theALIVE study (1988-1989). Baseline visit refers to the patient’sfirst visit after the start of this prospective analysis of liverenzyme measurements, starting in January 1995. Body mass indexwas assessed on a subset of participants consisting of all HIV-positive members (N = 462) and HIV-negative controls (N = 131). HIV-negative participants with body mass index data were similar to other HIV-negative subjects in age, race, and drug-use indicators, but differed (P > .05) in that they were more likely to be female and less likely to have an elevated ALT. Potential differences in HIV-negative participants with body mass index data means that the data may not be representative of the entire cohort, but should not substantially affect the correlation with ALT levels.
Statistical Analysis. The distribution of transaminase values was examined by scatter plots, and statistical inferences of medians were made according to HCV status using the Kruskal-Wallis test. Kaplan-Meier survival (time-to-event) curves were used to evaluate the natural history of ALT according to the first measurement. To assess correlates of serial ALT results, individual participants were categorized as follows: all normal ALT values, <50% elevated ALT values, ![]() 50%elevated ALT values, and always-elevated ALT values. Mean transaminase values were presented to allow assignment of confidence intervals. To exclude effects of extreme values, all analyses also were performed using medians, and no differences were detected when compared with analysis of mean values. Given that ALT is the transaminase measurement widely accepted and used in studies of treatment and outcomes in HCV infection, ALT was used solely in the rest of the analysis. Univariate comparisons between the four groups for dichotomous variables were made using the Mantel-Haenszel
50%elevated ALT values, and always-elevated ALT values. Mean transaminase values were presented to allow assignment of confidence intervals. To exclude effects of extreme values, all analyses also were performed using medians, and no differences were detected when compared with analysis of mean values. Given that ALT is the transaminase measurement widely accepted and used in studies of treatment and outcomes in HCV infection, ALT was used solely in the rest of the analysis. Univariate comparisons between the four groups for dichotomous variables were made using the Mantel-Haenszel ![]() 2test. Relationships between patient correlates and ALT values were further assessed by fitting multivariate logistic regression models of all factors significant in the univariate model at a significance of P < .05, comparing correlates of elevated ALTvalues with those of persistently normal ALT values. Body massindex was categorized into quartiles.
2test. Relationships between patient correlates and ALT values were further assessed by fitting multivariate logistic regression models of all factors significant in the univariate model at a significance of P < .05, comparing correlates of elevated ALTvalues with those of persistently normal ALT values. Body massindex was categorized into quartiles.
RESULTS
Study Sample. In January 1995, 1,306 individuals remained in the ALIVE cohort that was recruited in 1988 through 1989; HCV serology and liver enzyme testing was available for 1,235, and 1,164 (94%) were HCV-infected. The transaminase values from the HCV-infected individuals were compared with the 71(6%) HCV-uninfected individuals, who were then excluded from further analysis. Also excluded from further analysis were 122 HCV-infected individuals who had only one transaminase assessment. For the remaining 1,042 HCV-infectedindividuals, a total of 3,584 serial measurements were performed during 25 months of follow-up, a median of four per patient (range, 2-5 measurements).
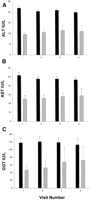
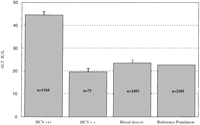
Transaminases and HCV Infection. ALT, AST, and GGT values were persistently higher in HCV-infected individuals than in HCV-uninfected individuals (Fig. 1).Because of its conventional use in the management of HCV infection,only the ALT values were considered further. At baseline, 38%of the HCV-infected group had ALT values above the normal range,while only 7% of the HCV-uninfected group had elevated values.The distribution of ALT levels of blood donors and commerciallaboratory controls was similar to HCV-uninfected study individuals,but lower than the HCV-infected study individuals (Fig. 2). The mean ALT values of the blood donors and reference laboratory controls were 23.6 IU/L and 22.7 IU/L, respectively.
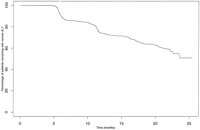
Natural History of ALT Values in HCV-Infected Individuals. At baseline, 647 (62%) of HCV-infected individuals had normal ALT levels. However, over 25 months of follow-up, 323 (49%) of these with initially normal ALTs had at least one elevated value (Fig. 3). Because clinicians may have more than one ALT measurement at the time of a patient’s initial evaluation, the predictive value of consecutive normal ALT measurements was also evaluated. Among individuals whose first two ALT values were normal, 16% had an elevated ALT on the third visit, while when the first three ALT values were normal, 11% had an elevated ALT on the fourth visit.
| Fig. 3. Time to first elevated ALT value in 647 HCV-infected patients with an initial normal ALT value. |
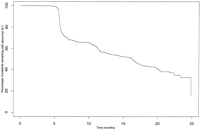
At baseline, 395 (38%) HCV-infected individuals had an elevated ALT value. However, over the 25 months of follow-up in this group, 332 (84%) had at least one normal ALT value (Fig. 4). Among individuals whose first two ALT values were elevated, 19% had a normal ALT on the third visit, while when the first three ALT values were elevated, 16% had a normal ALT on the fourth visit.
| Fig. 4. Time to first normal ALT value in 395 HCV-infected patients with an initial elevated ALT value. |
Overall, of the individuals with four or more ALT evaluations, 42% had persistently normal values, 15% had persistently elevated values, and 43% of individuals had intermittently elevated ALTs.
Correlates of Elevated ALT Measurements. ALT values were higher in HCV-infected individuals who were male, <41 years of age, used alcohol, who did not acknowledge current injection drug use, and who had a higher body mass index (Tables 1 and 2). An association with normal ALT levels was also found for other measures of drug use, including more frequent use, injection use of cocaine, and injection use of heroin (data not shown). No correlation was detected between ALT values and HIV infection or HIV-related immunosuppression as measured by CD4 count. ALT values were also not correlated with race, methadone use, marijuana use, tobacco use, number of sex partners, sexual orientation, or hepatitis B surface antigen status (data not shown).
| table 1. Correlates of Serial ALT Values Among HCV-Infected Individuals |
| table 2. Association of Body Mass Index With ALT |
In a multivariate model, male gender, alcohol use, body mass index, and age <41 years all remained associated with elevated ALT levels, while current injection drug use was negatively associated (table 3).
| table 3. Correlates of HCV-Infected Individuals With Elevated ALT Values: Adjusted Odds Ratios Estimated by Multivariate Logistic Regression |
Of 1,002 participants who had HCV-RNA testing and ALT testing on the same specimen, 879 (87.7%) had detectable RNA. ALTs wereelevated in 17 (13.8%) of 123 HCV-RNA-negative participants compared with 366 (41.6%) of 879 HCV-RNA-positive participants (P < .001).
DISCUSSION
ALT measurements are used to manage HCV-infected individuals. In fact, the 8- to 12-week ALT response has been described as the most important predictor of a sustained response to interferon.6 In addition, liver biopsies are the standard of care in persons with elevated ALT levels, but remain controversial in those with normal ALT values.22 Nonetheless, the natural history of ALT levels is poorly understood. In this investigation, which was performed prospectively in a setting representative of the majority of HCV-infected patients in the United States, marked variations in the ALT levels were noted between semiannual visits. One half of patients with an initially normal ALT level had an abnormal result at the next visit. Even among those with two consecutive normal ALT levels, 27% ultimately had an abnormal result in the next 12 months. If patients were categorized as having a persistently normal ALT based on two consecutive visits and these data were generalized to the estimated 4 million HCV-infected persons in the United States, >500,000 persons classified as having “normal ALTs” would have an abnormal result within the next year. These data underscore the importance of long-term follow-up in assessing HCV-infected patients.
The high visit-to-visit variability in ALT measurements may explain the poor correlation of the ALT and liver histology reported in most other studies. ALT trends may have more predictive value than individual measurements. Mathurin et al. found significant differences in the histological progression of liver disease in patients with persistently normal ALT levels compared with matched controls with elevated ALTs.23 In a preliminary evaluation of 60 liver biopsies performed on randomly selected individuals in this cohort, there was a high, but incomplete, correlation between the first two ALT results and the histological stage of disease.24 However, more study is necessary to examine the correlation between long-term ALT trends and clinically important outcomes.
In this investigation, HCV-infected individuals had higher ALT, AST, and GGT levels than uninfected individuals, as found previously with HCV infection acquired through intravenous druguse25 or transfusion,26 and among blood donors.27-30 Although ALT levels in this study were higher in HCV-infected individuals, during the 25 months of follow-up, 82% (816) of individuals had at least one ALT value in the normal range. One explanation for the overlap in ALT levels between HCV-infected and uninfectedindividuals is that cutoff values defining elevated measurementsmay be too high. For the assay used in this study, an elevatedALT was defined as ![]() 40 IU/L by Olympus, the maker of the materials and reagents, based on measurements of 200 New York blood donors. The cutoff value for an elevated ALT was also independently assessed by the Baltimore reference lab and was defined as greater than the ALT of 95% of persons without recognized disease. While many laboratories define elevated ALT values as greater than 97.5% of apparently normal individuals, this practice probably means that HCV-infected individuals are included as normal, because approximately 1.9% of the American population are infected, mostly without symptoms.31
40 IU/L by Olympus, the maker of the materials and reagents, based on measurements of 200 New York blood donors. The cutoff value for an elevated ALT was also independently assessed by the Baltimore reference lab and was defined as greater than the ALT of 95% of persons without recognized disease. While many laboratories define elevated ALT values as greater than 97.5% of apparently normal individuals, this practice probably means that HCV-infected individuals are included as normal, because approximately 1.9% of the American population are infected, mostly without symptoms.31
This observation suggests that lowering the threshold for elevated ALT values may improve the correlation of ALT and HCV status. However, in this investigation, no such clinically useful change could be identified. For example, if the upper limit of a normal ALT was reduced to 33 IU/L, the percentage of ALT measurementsreclassified as elevated would increase from 37% to 50% in HCV-infected individuals. However, this change also would increase the percentage of ALT measurements classified as elevated from 8% to 13% in HCV-uninfected individuals. Further study would be needed to ascertain if ALT values in this range reflect disease or another clinically important outcome.
In the HCV-infected individuals, ALT levels decreased with increasing age, a trend not seen in HCV-uninfected individuals. In this cohort, older age correlated with longer duration of HCVinfection.18 These findings may indicate that, on average, ALT levels decrease as HCV infection persists, as has been found by other investigators.32
In this investigation, subjects who ingested alcohol daily in the 6-month interval before the ALT assessment were more likely to have persistently elevated ALT values, though a dose-responserelationship was not detected. This finding remained after adjustingfor gender, drug use, and other factors. No association betweenalcohol use and serum ALT levels was detected by Piton et al.among HCV-infected and HCV-uninfected individuals.33 However, their study involved a smaller number of HCV-infected persons, and in most studies, alcohol use is associated with more severe liver histology.34-39
In this study, current intravenous drug use was associated with lower ALT levels, an effect that was independent of age, body mass index, and duration of injecting over time. Slower histological progression of HCV infection among injection drug users compared with transfusion-associated individuals has been reported in anotherstudy.40 The ALT level is presumed to be a marker of hepatic inflammation and, in well-characterized mouse models of hepatitis B virus infection, correlates with cell death by apoptosis.41 Drug use-related reduction in immune response to HCV may explain this inverse association. Studies of other groups of relatively immunosuppressed HCV-infected individuals, e.g., HCV-infected hemodialysis patients and renal transplant recipients, have demonstratedlower-than-expected transaminase levels.42,43 Alternatively, injection drug users could be nutritionally deficient, causing the ALT assay to be falsely low, as has been seen in alcoholics with pyridoxal phosphate deficiency.31 At present, too littleis known about the pathogenesis of HCV infection to further explicate the inverse association of drug use and serumALT.
It was anticipated that ALT levels in this community-based study might be lower than those from liver clinics, because the latter generally represent patients with clinically significant disease. In addition, the route of infection may be important, because, as mentioned above, infection following transfusion appears to be more severe than that from drug use. Even in this study, active drug use was associated with lower ALT levels. Thus, the results of this study may not be applicable to liver clinic-based cohorts or patients infected by transfusion. However, these trends reflect the natural history of most HCV infections in developed countries, because they occur in the context of illicit drug use. Indeed, Alter et al. found substantial ALT variability in their study of the natural history of community-acquired hepatitis C in the United States.8
In this investigation, higher ALT levels were found in participants with a higher body mass index, as was found in the study by Pitton et al.33 However, no association was detected with HIV infection or HIV immunosuppression as measured by the CD4 lymphocyte count. This was unexpected, because HIV infection is associated with increased HCV viral load and an accelerated course of disease.44,45
In conclusion, marked variability in ALT measurements was noted in HCV-infected individuals. Given the number of HCV-infected individuals worldwide and the increasing development of novel treatment strategies, further study is necessary to evaluate the clinical importance of long-term ALT trends in HCV-infected individuals.
Acknowledgment
The authors thank Quest Diagnostics for the performance of transaminase determinations. The authors acknowledge Dr. William A. Meyer, III, and David Carder for their assistance in obtaining the ALT data from a reference population of samples submitted to a Baltimore commercial laboratory. The authors thank Dr. Paul Ness, Chief Executive Office of the Greater Chesapeake and Potomac Region, American Red Cross Blood Service, for his assistance in collecting the ALT data from Baltimore blood donors.
Abbreviations
Abbreviations: HCV, hepatitis C virus; ALT, alanine transaminase; HIV, human immunodeficiency virus; AST, aspartate transaminase; GGT, ![]() -glutamyl transferase.
-glutamyl transferase.
Footnotes
Received July 16, 1998; accepted October 14, 1998.
Supported in part by Public Health Service grants RO1 DA10627, U19 A140035, R01 DA08004, R01 DA04334.
Address reprint requests to: David L. Thomas, M.D., M.P.H., Division of Infectious Diseases, Johns Hopkins University, 1147 Ross Research Building, 720 Rutland Avenue, Baltimore, MD 21205. E-mail: dthomas@welchlink.welch.jhu.edu; fax: (410) 614-5138.
REFERENCES
Copyright © 1999 by the American Association for the Study of Liver Diseases